A lithium ion-selective electrode gave the potentials given next for the following standard solutions of LiCl and
Question:
A lithium ion-selective electrode gave the potentials given next for the following standard solutions of LiCl and two samples of unknown concentration:
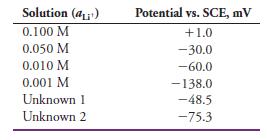
(a) Construct a calibration curve of potential versus log aLi+, and determine if the electrode follows the Nernst equation.
(b) Use a linear least-squares procedure to determine the concentrations of the two unknowns.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:





