A cylindrical catalyst pore of radius, (r_{0}), and length, (L), catalyzes two competing first-order reactions. [begin{array}{ll}a
Question:
A cylindrical catalyst pore of radius, \(r_{0}\), and length, \(L\), catalyzes two competing first-order reactions.
\[\begin{array}{ll}a \rightarrow b & r_{a}=-k_{a b}^{\prime \prime} c_{a} \\a \rightarrow c & r_{a}=-k_{a c}^{\prime \prime} c_{a}\end{array}\]
The binary diffusivities of \(a / c\) and \(a / b\) are \(D_{a c}\) and \(D_{a b}\) and we will assume they are independent of one another. If \(c\) and \(b\) are dilute, i.e., the reaction "conversion" is low, we saw in Chapter 2 that the diffusivity of \(a\) through the mixture can be written as:
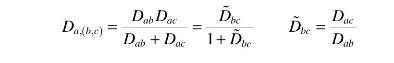
a. Derive the differential equation for the concentration of \(a\) in the pore.
b. Assuming the concentration of \(a\) at the pore mouth is \(c_{a 0}\), and the end of the pore is sealed off, solve the differential equation for the concentration of \(a\).
c. Determine the flux of \(a\) into the pore through the pore mouth and how that depends upon the diffusivity ratio and the reaction rates.
Step by Step Answer:






