A liquid mixture of diethyl ketone (1) and n-hexane (2) is fed into a flash distiller operating
Question:
A liquid mixture of diethyl ketone (1) and n-hexane (2) is fed into a flash distiller operating at 328 K and 0.4 bar. The feed rate is 2.15 mol/s and the feed is 50% (by mol) of the n-hexane.
A. What is the composition and amount of the resulting equilibrium phases?
B. If you take the exiting liquid phase from the flash distiller and send it into a second flash distiller operating at 328 K and 0.3 bar, what is the composition and amount of the resulting equilibrium phases? What percentage of the diethyl ketone in the initial feed entering the first flash distiller ends up in the liquid exiting the second flash distiller?
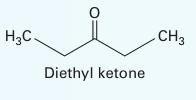
C. Which assumption that you made in solving this problem would you characterize as least valid and why?
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





