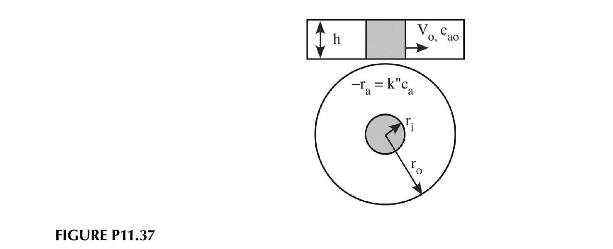
A radial flow reactor is often used for highly exothermic reactions. The high radial velocities at the
Question:
A radial flow reactor is often used for highly exothermic reactions. The high radial velocities at the reactor inlet compensate for any hot spots that might form in the reactor there. Consider the case where we are running a pseudo-first-order reaction in a radial reactor like that shown in Figure P11.37. Determine the concentration of \(a\) in the reactor as a function of radial position. The volumetric flowrate of reactant can be assumed constant and the inlet concentration is \(c_{a o}\). How would the results change if the reaction were second order in the concentration of \(a\) ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





