A tank contains a mixture of water and carbon dioxide. The mol fraction of CO 2 in
Question:
A tank contains a mixture of water and carbon dioxide. The mol fraction of CO2 in the liquid is 5.0 × 10−4 and the temperature is 70 °C.
a) Calculate the total pressure as well as the composition of the vapor phase.
b) To what temperature should you bring the system in order to increase the mol fraction of CO2 in the liquid by a factor of 5, if the total pressure is to remain constant?
c) Calculate the composition of the vapor phase for the conditions of part (b). Henry’s constant of CO2 is 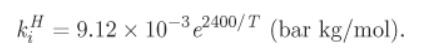
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9780132693066
1st Edition
Authors: Themis Matsoukas
Question Posted:





