A young engineer has the bright idea of trying to separate methanol and water by centrifugation as
Question:
A young engineer has the bright idea of trying to separate methanol and water by centrifugation as shown in Figure P2.24. The target system is an antifreeze consisting of $30 \mathrm{~mol} %$ methanol. The partial molar volumes and pure component molar volumes at $25^{\circ} \mathrm{C}$ are:
\[\begin{array}{ll}\overline{V_{m}}=38.632 \mathrm{~cm}^{3} / \mathrm{mol} & \overline{V_{w}}=17.765 \mathrm{~cm}^{3} / \mathrm{mol} \\V_{m}=40.727 \mathrm{~cm}^{3} / \mathrm{mol} & V_{w}=18.068 \mathrm{~cm}^{3} / \mathrm{mol}\end{array}\]
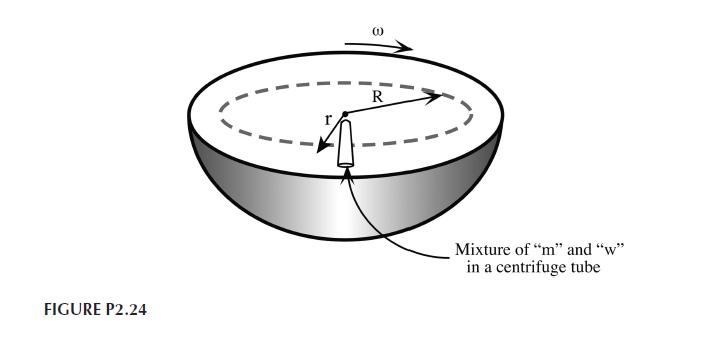
Assuming a centrifuge like that in Figure P2.24 operates at 20,000 rpm and a temperature of $25^{\circ} \mathrm{C}$, what would be the concentration of $m$ at $r=0.2 \mathrm{~m}$ ? What is the maximum separation ratio there; $x_{m L} / x_{w L}$ ? Assume the pressure gradient, $\partial P / \partial r=4 \pi^{2} \omega^{2} ho r$, where $\omega$ is the revolution frequency and $ho$ is the fluid density.
Step by Step Answer:






