Acetone can be converted into ketene and methane by a vapor-phase reaction: CH 3 COCH 3
Question:
Acetone can be converted into ketene and methane by a vapor-phase reaction:
CH3 COCH3 ↔ CH2 CO + CH4
Assume ΔCP = 0 for this process.
A. Since Appendix C contains no data for ketene, use the Joback method to estimate ΔHf0 and ΔGf0 for this compound.
B. Plot the equilibrium constant of this reaction, vs. temperature, from T = 25°C to T = 800°C.
C. A popular Chemical Reaction Engineering text (Fogler, 1992) contains an example in which this reaction is carried out at 1035 K. Based on part B, does 1035 K seem like a well-chosen temperature?
D. If a reactor has constant T = 1035 K and P = 1 bar, and is filled with pure acetone at the beginning, what fraction of the acetone is converted into products at equilibrium?
E. 1000 mol/min of pure acetone enters a steady state, adiabatic flow reactor at T = 1035 K and P = 1 bar, and the stream exiting the reactor is at P = 1 bar and at equilibrium. What is the temperature and composition of the stream leaving the reactor?
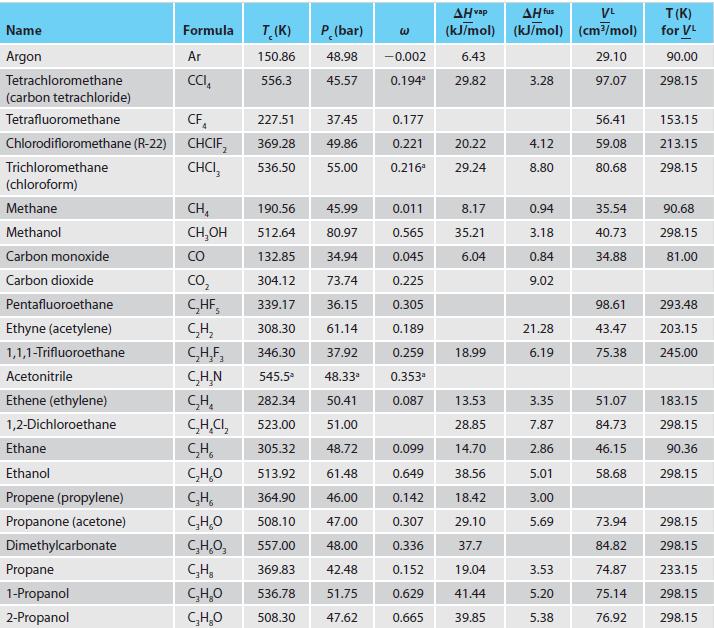
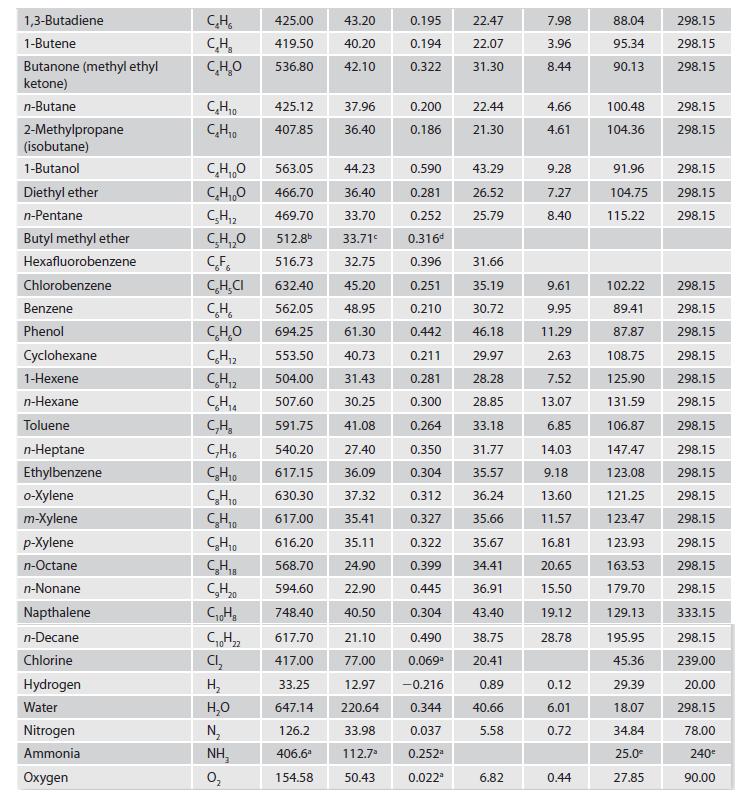
Appendix C.
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco




