At 80F, we mix 10 lb m of sulfuric acid with 20 lb m of water. What
Question:
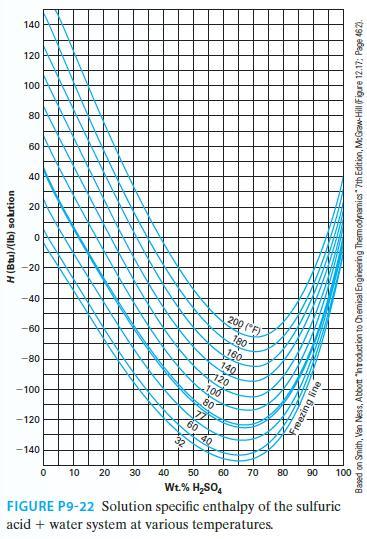
At 80°F, we mix 10 lbm of sulfuric acid with 20 lbm of water. What is the resulting heat of mixing for this process? Is the heat liberated or absorbed? Use Figure P9-22.
Transcribed Image Text:
H (Btu)/(lb) solution 140 120 100 80 60 40 20 O -20 -40 -60 -80 -100 -120 -140 200 (F) 180- 100 KAN gline 0 10 20 30 40 50 60 70 80 90 100 Wt.% H₂SO4 FIGURE P9-22 Solution specific enthalpy of the sulfuric acid + water system at various temperatures. Based on Smith, Van Ness, Abbott "Introduction to Chemical Engineering Thermodynamics" 7th Edition, McGraw-Hill (Figure 12.17: Page 462).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
A domestic common camer has the following data on gross receipts and expenses for the first quarter of 2018: TV Transport of passengers Transport of goods and cargoes Expenses, transport of...
-
A mass of 500 lb m of 40 wt% sulfuric acid solution at 140F is diluted with 200 lb m of pure water at 100F. What is the concentration of the resulting solution? What is the heat effect (liberated or...
-
The senior management at Davis Watercraft would like to determine if it is possible to improve firm profitability by changing their existing product mix. Currently, the product mix is determined by...
-
In Problems 1968, solve each equation, if possible. -4 x + 4 || -3 x+6
-
Describe circumstances and give an example of when free cash flows to equity shareholders and free cash flows to all debt and equity stakeholders will be identical. Under those circumstances, will...
-
What are the major tools for valuation, and how would they be different from each other?
-
What are some common reasons for resistance to control?
-
For the following highway network system, determine the maximal flow in vehicles per hour: The highway commission is considering adding highway section 34 to permit a flow of 2000 vehicles per hour...
-
Write a Java conditional statement that implements the following table, where grade is an integer and school is a String. Both variables have been declared and grade has been initialized to a value...
-
Estimate the partial molar enthalpy of sulfuric acid at 140F at the following two compositions using Figure P9-22. A. 30% by wt sulfuric acid B. 80% by wt sulfuric acid H (Btu)/(lb) solution 140 120...
-
Using tabulated experimental data from the literature for either the excess molar volume or excess molar enthalpy of a system of your choice, provide the following information. A. A plot of the...
-
You are using exponential smoothing on an annual time series concerning total revenues (in $millions). You decide to use a smoothing coefficient of W = 0.20, and the exponentially smoothed value for...
-
Which of these is also called T-group training? Role enactment In-basket training Sensitivity training Simulation training
-
In which of the following situations decision-making is easy? You have complete information You are new to a domain You are an expert in a domain Market is fluctuating
-
Which organizational structure would you prefer to work on as an employee? Least preferred? Why?
-
How can you evaluate the effectiveness of training?
-
In which model does an individual seek to make decisions with an aim to satisfy their own or an organizations interests or goals? Administrative model Political model Statistical model Classical...
-
Find the present value of a 3-year, $20,000 ordinary annuity deposited into an account that pays 12% annual interest, compounded monthly. Solve for the present value of the annuity in the following...
-
1. Using the information from Problem 16-4B, prepare a statement of cash flows for Lim Garden Supplies Inc. using the direct method of presenting cash flows from operating activities. 2. How does Lim...
-
Explain what is wrong with the following statement: At its isoelectric point, the charge on all molecules of a particular protein is 0.
-
Calculate the pH at each of the following points in the titration of 50.00 mL of 0.010 0 M NaOH with 0.100 M HCl. Volume of acid added: 0.00, 1.00, 2.00, 3.00, 4.00, 4.50, 4.90, 4.99, 5.00, 5.01,...
-
Calculate the pH at each point listed for the titration of 50.0 mL of 0.050 0 M formic acid with 0.050 0 M KOH. The points to calculate are V b = 0.0, 10.0, 20.0, 25.0, 30.0, 40.0, 45.0, 48.0, 49.0,...
-
A metal disk of radius R, thickness d, and conductivity lies in the x-y plane and is cantered at the origin. There is a time-dependent, uniform magnetic field permeating the region. (a) Determine...
-
On 4 th May 2022, Tom invested in 200 shares of Grow Crop using the margin trading facility provided by his broker. The broker requires an initial margin of 60% and a maintenance margin of 50%. The...
-
You have a savings goal of $4500 in 5 years. You find an investment option with a 6% per annum rate, compounded monthly. How much do you need to invest today in order to reach your savings goal?

Study smarter with the SolutionInn App


