Consider the density of the mixture 1,2-dicholoro ethane (1) + cholorobenzene (2) at 298.15 K. Use Table
Question:
Consider the density of the mixture 1,2-dicholoro ethane (1) + cholorobenzene (2) at 298.15 K. Use Table P9-26 to answer the following questions
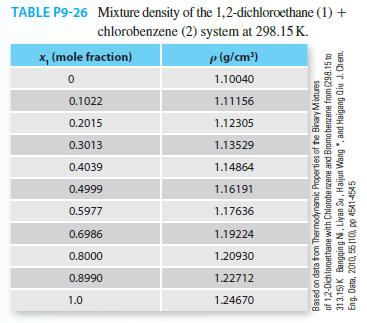
A. Does this mixture behave as an ideal solution?
B. If you mixed 100 ml of both components to gether at 25°C, will you have more or less than 200 ml? What percentage different from the ideal solution value is the experimental value?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





