Consider the following experimental data in Table E11-11 for the ethanol (1) + 2, 2, 4-tri methyl
Question:
Consider the following experimental data in Table E11-11 for the ethanol (1) + 2, 2, 4-tri methyl pentane (2) system at 333.15.
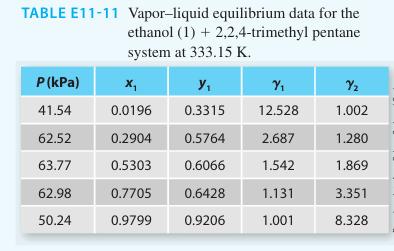
Considering the values of the activity coefficients, you know that this system will not behave as an ideal solution. However, would the 1- parameter Margules equation be a reasonable model to account for the liquid phase non-idealities? Why or why not? Explain your answer based on the information provided in the table.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





