Consider the pentafluorethane [R-125] (1) + isobutane (2) system at 30C. Using a gamma-phi modeling approach, calculate
Question:
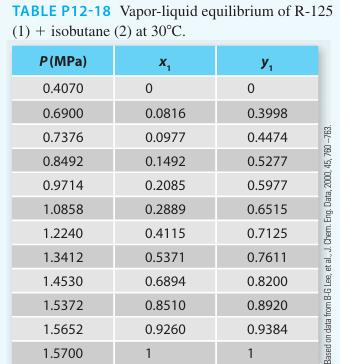
Consider the pentafluorethane [R-125] (1) + isobutane (2) system at 30°C. Using a gamma-phi modeling approach, calculate the Pxy diagram for the system using the 2-parameter Margules equation and the virial equation. Compare the predicted values with the experimental data provided in Table P12-18 (on the same plot). Also please provide two additional modeling approaches to the plot.
1. Model the system using modified Raoult’s Law (ideal gas for the vapor). This includes calculating the activity coefficients assuming an ideal gas for the vapor (as in Chapter 11).
2. Model the system using Raoult’s Law.
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:




