Consider the transfer of substance (a) between water (1) and isooctane (2). The two solvents are immiscible,
Question:
Consider the transfer of substance \(a\) between water (1) and isooctane (2). The two solvents are immiscible, and both can be assumed to extend out toward infinity. The concentrations of \(a\) in both solvents are very small so that we can write the transient diffusion equations in both phases in terms of the concentration of \(a\).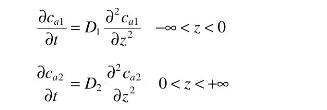
There is also an equilibrium partition coefficient between the two phases so that \(c_{a 2}=m c_{a 1}\). Initially, the concentrations in the two phases are \(c_{a 1 o}\) and \(c_{a 20}\).
a. What are the boundary conditions for this problem?
b. Solve the equations (refer to Section 6.5.1) and determine the concentration gradients.
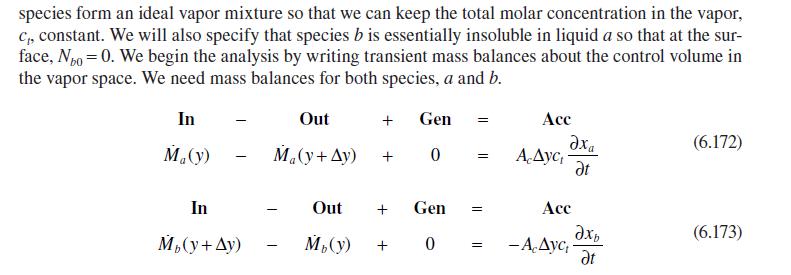
c. We can specify the transfer of \(a\) (flux) from water to isooctane in terms of a mass transfer coefficient, \(k_{c}\), and the concentration difference in the isooctane phase, \(\left(c_{a 2}(0)-c_{a 2}(\infty)\right)\). Determine the instantaneous mass transfer coefficient.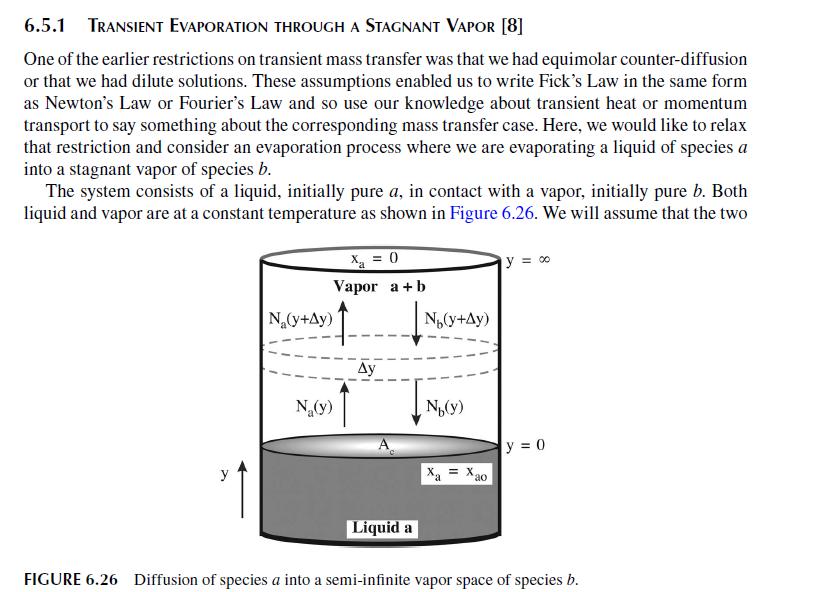
Step by Step Answer:






