Find the molar volume of nitrogen at P = 10 bar and T = 330 K, using
Question:
Find the molar volume of nitrogen at P = 10 bar and T = 330 K, using the following methods.
A. The Soave equation of state
B. The Peng-Robinson equation of state
C. The virial equation of state
D. The Lee-Kesler generalized correlation
E. Appendix F
Appendix F
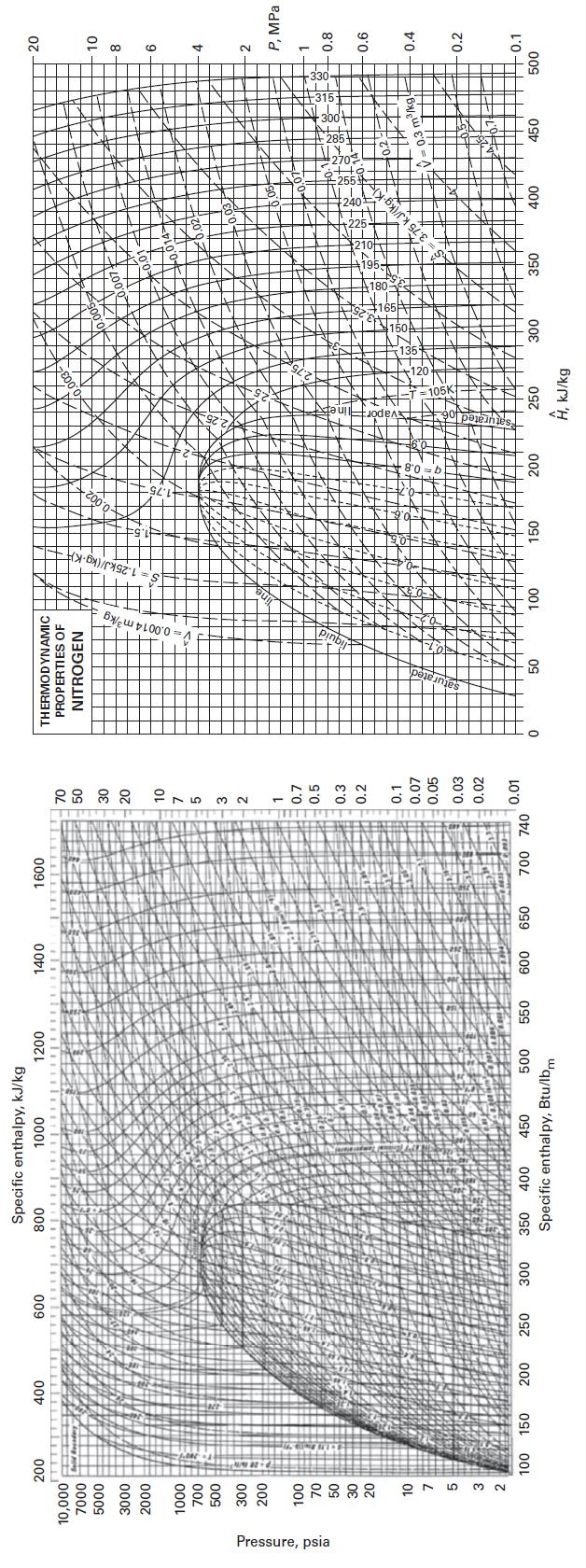
Transcribed Image Text:
Pressure, psia 200 10,000 7000 5000 3000 2000 1000 700 500 300 200 100 70 50 30 20 10 3 2 100 150 400 200 600 250 300 Specific enthalpy, kJ/kg 1000 800 1200 350 400 450 500 Specific enthalpy, Btu/lbm 550 1400 600 650 1600 700 740 70 50 30 20 10 7 5 3 2 1 0.7 0.5 0.3 0.2 0.1 0.07 0.05 0.03 0.02 0.01 0 THERMODYNAMIC PROPERTIES OF NITROGEN 50 <> 100 150 200 250 Ĥ, kJ/kg 300 350 400 0.3 m³/kg 450 20 10 8 6 4 2 P, MPa 1 0.8 0.6 0.4 0.2 0.1 500
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Find the molar volume of Freon 22 at P = 5 bar and T = 20C (it is a vapor at these conditions), using the following methods. A. The Soave equation of state B. The Peng-Robinson equation of state C....
-
Find the molar volume of methane at P = 15 bar and T = 200C (it is a gas at these conditions), using the following methods. A. The Soave equation of state B. The Peng-Robinson equation of state C....
-
Question 3. Find the second derivative of y = x - 1 Question 4. If f(x) = x-1 Find f'(-2).
-
Which one of the following results from the latest decision round are least important in providing guidance to company managers in making their strategic moves and decisions to improve their...
-
Foreign exchange rates are used to establish budgets and track actual performance. Of the various exchange rate combinations mentioned in this chapter, which do you favor? Why? Is your view the same...
-
One week in lab, youre given a spring-loaded bar that can be used to strike a metal ball. Your assignment is to measure what size impulse the bar delivers to the ball. You and your lab partner decide...
-
What pressure, \(p_{1}\), is needed to produce a flowrate of 0.09 \(\mathrm{ft}^{3} / \mathrm{s}\) from the tank shown in Fig. P3.92? Figure P3.92 Air P1 T Gasoline 2.0 ft Salt water SG = 1.1 0.06-ft...
-
Compute the price of an American call option on the same ZCB of the previous three questions. The option has expiratio t =6 and strike = 80 K Term Structure Lattice 2 r(0,0) 5.00% 3 1.10 0.9 0.50 b1...
-
If following code was executed, what is the final value of variable y? y = 100; m = 70; if m < 50 y = y + m; end
-
Use the Joback method to estimate T c and P c of each of the following compounds. A. Butane B. 1-Hexanol C. 2-chloropentane D. 3-Hexene E. 1,3-butadiene
-
Using data in Appendix C-1, determine the Peng-Robinson parameters a and b for each of the following compounds at the temperature T = 100C. A. Ethane B. Acetone C. Benzene D. Toluene E. Decane Name...
-
Using the answer to Problem 20 on Intel growth rates and the current trading price, determine the current required rate of return for the company.
-
Mandy, a CFP professional, used credit bureau information to complete a review for her client Annie. The Credit bureau is a private organization that keeps track of individuals credit history a...
-
1. Consider the following two-dimensional, incompressible flow through the converging channel as shown The mean velocity at x-0 in Figure 1. Assume the cross-section area per unit depth is A = is V...
-
Jared expects to be disabled for 11 months. His benefits will be for 14 months for a total of $ . Jared receive benefits because he will not be disabled long enough to qualify for them. (Hint: If...
-
Explain three asset pricing anomalies that exist in cross-section of stock returns, and describe the corresponding strategies.
-
A sole trader Blake, operating company ABC has some business losses, and as a result does not have enough money to pay for a supplier. As a result, he invests an additional $ 100,000 on 28 March...
-
As a manager at RHC, LLP, part of Charlies tasks include drafting the audit plan for his audit clients, including determining the appropriate audit procedures to be performed over the course of the...
-
Find the image of x = k = const under w = 1/z. Use formulas similar to those in Example 1. y| y = 0 -21 -2 -1 -1, /1 12 T -1 -1 y= -2 x =0
-
Suppose that data show that a certain stock price is normally distributed with a mean of $150 and a variance of 100. Create a simulation to compare the results of the following two strategies over...
-
Write a script le to simulate 100 plays of a game in which you ip two coins. You win the game if you get two heads, lose if you get two tails, and ip again if you get one head and one tail. Create...
-
Write a script le to play a simple number guessing game as follows. The script should generate a random integer in the range 1, 2, 3, . . . , 14, 15. It should provide for the player to make repeated...
-
Bob believes he can save $175,000 to use when his son begins college in 12 years. Assume Bob currently has no savings. If he saves $1,000 per month, what is the estimated annual investment rate...
-
Chad Williams, CEO of Red Cloud Mining, a Toronto based consultancy to the mining sector has a challenge to overcome. A client is seeking his company's assistance to place a value on a non-producing...
-
At December 31, 2025, Sarasota Company has outstanding noncancelable purchase commitments for 39,500 gallons, at $3.42 per gallon, of raw material to be used in its manufacturing process. The company...

Study smarter with the SolutionInn App


