In Example 12-2 in this chapter, you evaluated two equation of state approaches for the prediction of
Question:
In Example 12-2 in this chapter, you evaluated two equation of state approaches for the prediction of the phase behavior for the propane (1) + n-butane (2) system at 323.15 K. Once you completed this chapter, being an inquisitive student, you wondered if this system could also be modeled by treating the liquid phase as an ideal solution and the vapor phase as an ideal solution (since the system pressure was over 15 bar). Explore your curiosity by providing the following modeling approaches for this system on the same plot:
A. Raoult's Law (ideal solution for the liquid; ideal gas for the vapor)
B. Ideal Solution—Ideal Solution (ideal solution for the liquid, but include the impact of pressure; ideal solution for the vapor–virial equation is your model).
Compare your results with the experimental data plotted as symbols
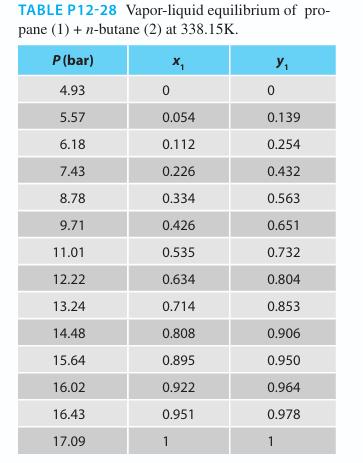
Example 12-2.
You are a student doing a project where you are evaluating process options in the separation of a propane (1) + n-butane (2) system at 323.15 K. There are choices of equations of state that you can use and you need to make a selection. Prior to this, you decide to predict the phase behavior yourself using two of the choices: the van der Waals equation of state and the Peng-Robinson equation of state. Provide the Pxy diagram for this system using both models.
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco




