In Section 3.4, we discussed briefly the idea of surface diffusion. We can use an expression very
Question:
In Section 3.4, we discussed briefly the idea of surface diffusion. We can use an expression very similar to equation (3.30) derived from kinetic theory to look at surface diffusion of gases adsorbed on solid surfaces.
\[D_{o}=\frac{1}{4} v \lambda_{s}^{2} \sim \frac{1}{4} v_{s} \lambda_{s}\]
The idea is that gas molecules adsorb onto a surface and move from place to place by hopping from one favorable location to another. The frequency of hops is \(v\) and the distance covered by each hop is \(\lambda_{s}\). The gas molecules are attracted to the surface by strong intermolecular forces and so there is an activation barrier that must be overcome for the molecules to move. This energy barrier is called the isosteric heat of adsorption, \(q_{s t}\), and so the full value of the diffusivity can be represented as:
\[D=D_{o} \exp \left[-\frac{a q_{s t}}{R T}\right]\]
a. The following table shows data of \(D\) vs \(q_{s l} / R T\) for carbon dioxide on porous silica at \(-50^{\circ} \mathrm{C}\). Using a semi-log plot, graph the data and determine the constants \(a\) and \(D_{o}\).
b. How do the magnitudes of these diffusivities compare with the diffusivity of carbon dioxide in water?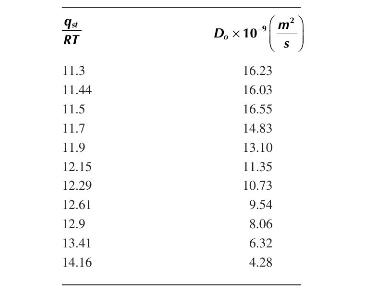
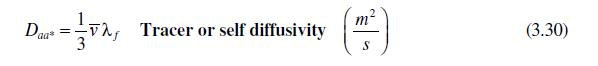
Step by Step Answer:





