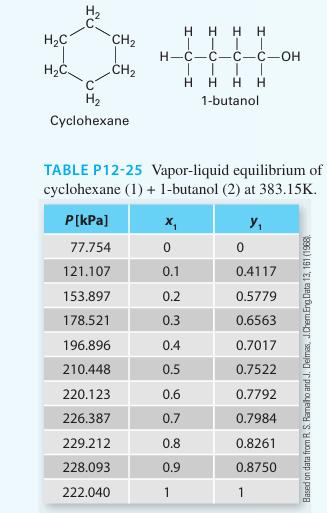
Predict the Pxy behavior for a mixture of cyclohexane (1) + 1-butanol (2) at 383.15 K using
Question:
Predict the Pxy behavior for a mixture of cyclohexane (1) + 1-butanol (2) at 383.15 K using the Peng-Robinson equation of state. Compare the predictions to the experimental data given in Table P12-25. Determine a “best fit” binary in teractionparameter (k12 ) that best matches the equation of state pressures to the experimental pressures. Plot those new predictions on the same curve. Comment on your results.
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:




