Resolve Example Problem 11.1, but now include the nitrogen gas into the system. Note the Henrys Law
Question:
Resolve Example Problem 11.1, but now include the nitrogen gas into the system. Note the Henry’s Law constants for nitrogen in water in Table P11-15:

Transcribed Image Text:
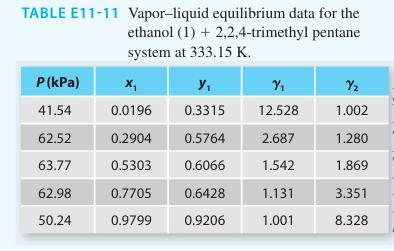
TABLE E11-11 Vapor-liquid equilibrium data for the ethanol (1) + 2,2,4-trimethyl pentane system at 333.15 K. P (kPa) 41.54 62.52 63.77 62.98 50.24 x₁ Y₁ 0.0196 0.3315 0.2904 0.5303 0.7705 0.9799 0.5764 0.6066 0.6428 0.9206 Y₁ 12.528 2.687 1.542 1.131 1.001 Y₂ 1.002 1.280 1.869 3.351 8.328
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The Henrys law constant for O 2 in water at 25C is given in Table 13.2. Which of the following is a reasonable constant when the temperature is 50C? Explain the reason for your choice. (a) 6.7 10 4...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
In Exercises 7681, find the domain of each function. g(x) = 4 x - 7
-
The Coca-Cola Company is a global soft drink beverage company (ticker symbol = KO) that is a primary and direct competitor with PepsiCo. The data in Exhibits 12.1312.15 include the actual amounts for...
-
Ella Mendes prepared the following trial balance for her yoga studio, Mindful Meditation, for the year ended December 31, 2021. Instructions Use the trial balance to do the following: a. Prepare an...
-
The channel in Fig. P10.48 has two floodplains as shown. Find the discharge if the center channel is lined with brick and the two floodplains are lined with cobblestones. The slope \(S_{0}\) is...
-
1. What are the arguments in favor of Manufacturing Vice President Moores proposal to purchase the manufacturing software from EMS? 2. How should BMC obtain the needed manufacturing software? 3. What...
-
This is a practical activity which will require you to resolve a conflict between two or more employees. You will need to research and utilise appropriate sources of internal and external assistance....
-
Which system provided here, if any, would be best modeled by an ideal solution? If any of the solutions are non-ideal, discuss whether the Scatchard Hildebrand approach would be appropriate to model...
-
An experiment on the vapor-liquid equilibrium for the methanol (1) + dimethyl carbonate (2) system at 337.35 K provides the following information (S. Yunhai et al., 2005): x 1 50.0, y 1 50.0 and P =...
-
Verify that if the expected cell frequencies are calculated in accordance with the rule on page 372, their sum for any row or column equals the sum of the corresponding observed frequencies.
-
With what group of others do you most closely identify? Those from your home town? Those of your same sex? Religion? Race? Income level? IQ level? Who are the people with whom you feel most relaxed?...
-
Users complain that a database server has slowed down over the last hour despite the fact that no more users have connected Option. You run an antimalware scan, which comes up clean Option. The...
-
You have been asked to determine the NPV of a project. The has a cost of $800. This cost is incurred at year O. The expected cash flow in year 1 is $130. The expected cash flow in year 2 is $220. The...
-
Pops Popcorn has three project choices for the coming year, but only $9,000 in its budget for new projects. Project 1 is a new corn seed separator that identifies grannies (seeds that do not pop when...
-
A fill-up for a contractors truck cost $87.17. If the tank took 28.13 gal of gas, what was the price per gallon? Give answer to the nearest tenth of a penny (3 places after the decimal). Cut boards...
-
Approximately 76% of baby boomers aged 43 to 61 are still in the workforce (The Boston Globe, July 10, 2008). Six baby boomers are selected at random. a. What is the probability that exactly one of...
-
Revol Industries manufactures plastic bottles for the food industry. On average, Revol pays $76 per ton for its plastics. Revol's waste-disposal company has increased its waste-disposal charge to $57...
-
A solution contains 63 different conjugate acid-base pairs. Among them is acrylic acid and acrylate ion, with the equiliborium ratio [acrylate]/[acrylic acid] = 0.75. What is the pH of the solution?...
-
Find the pH of a solution prepared by dissolving 1.00 g of glycine amide hydrochloride (Table 8-2) plus 1.00 g of glycine amide in 0.100 L. Glycine amide C;H&N20 H,N. FM 74.08 NH,
-
(a) Find the pH of a solution prepared by dissolving 1.00 g of glycine amide hydrochloride (Table 8-2) plus 1.00 g of glycine amide in 0.100 L. (b) How many grams of glycine amide should be added to...
-
2. YA Y F FA 12 C 0=60 X In the figure, XY-coordinate system is the global coordinate System, and xy-coordinate system is the local coordinate system. The force vector at C on truss element is given...
-
A search tool finds online information based on criteria you specify or selections you make. Search tools include search engines and search boxes on webpages. The more effectively you use search...
-
Stuart Daniels estimates that he will need $ 2 2 , 0 0 0 to set up a small business in 7 years. ( a ) How much ( in $ ) must Stuart invest now at 8 % interest compounded quarterly to achieve his goal?

Study smarter with the SolutionInn App


