The gas phase reaction oxidation of SO 2 to SO 3 is carried out at a pressure
Question:
The gas phase reaction oxidation of SO2 to SO3 is carried out at a pressure of 1 bar with 20% excess air in an adiabatic reactor. The reactants enter at 25 °C and equilibrium is obtained at the exit of the reactor.
a) Write the balanced equation for the formation of one mole of sulfur trioxide.
b) What is the heat of reaction of the equation in part (a)?
c) Determine the composition and the temperature of the product stream from the reactor.
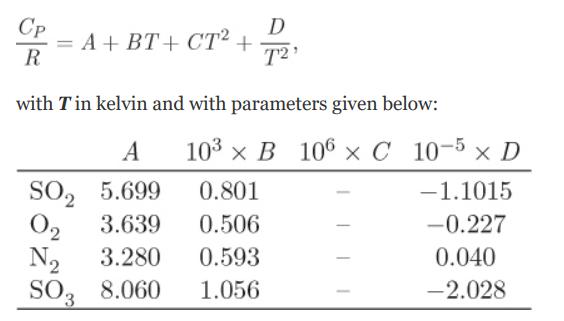
The heat capacities of the components are given by the equation.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9780132693066
1st Edition
Authors: Themis Matsoukas
Question Posted:





