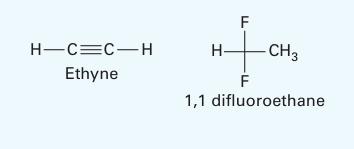
Use a - approach to model the vapor-liquid equilibrium of an ethyne [acetylene] (1) + 1, 1
Question:
Use a - approach to model the vapor-liquid equilibrium of an ethyne [acetylene] (1) + 1, 1 difluoro ethane {R-152a] (2) system at 303.2 K. Treat the
liquid using the 2-parameter Margules equation and the vapor as an ideal solution (described by the virial equation). Report the following:
∎ Raoult’s Law predictions
∎ Modified Raoult’s Law predictions (ideal gas for the vapor phase)
∎ Υ-Φ modeling results
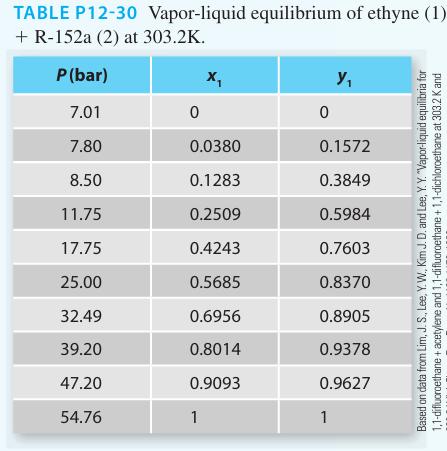
∎ Experimental data as symbols given in Table P12-30
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





