Using the NIST Webbook, if one looks up the molar enthalpy of pure benzene at 308.15 K
Question:
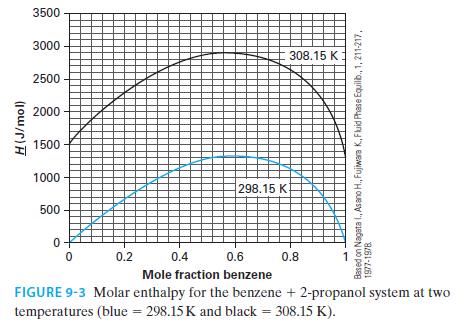
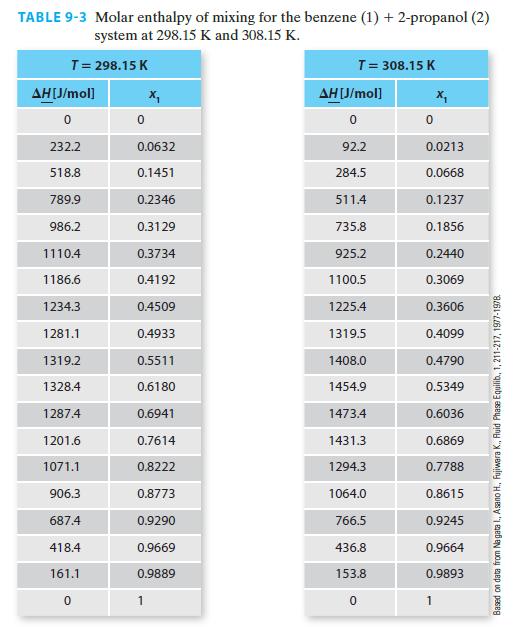
Using the NIST Webbook, if one looks up the molar enthalpy of pure benzene at 308.15 K and 1 bar, the reported value is –6359.6 J/mol. Figure 9-3 has this same property as being equal to 1356.9 J/mol (via Example 9-2). Whose value, if any, is in error? Please explain your answer.
Transcribed Image Text:
H(J/mol) 3500 3000 2500 2000 1500 1000 500 0 0 298.15 K 0.2 308.15 K 0.4 0.6 Mole fraction benzene FIGURE 9-3 Molar enthalpy for the benzene + 2-propanol system at two temperatures (blue = 298.15 K and black = 308.15 K). X 0.8 Based on Nagata I., Asano H., Fujiwara K., Fluid Phase Equilib., 1, 211-217, 1977-1978 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Suppose that the depreciation of EA/B was 7% over the past year, and prices in country A increased by 4% from this time last year. Assuming relative PPP holds, what was the rate of inflation in...
-
Marili had a full life with her husband, Jack and their 3 adult daughters (Jackie, Sophie, and Stephanie). Jackie has 2 children and Stephanie had 2 children. Unfortunately, Stephanie passed away 2...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Why does Erasmus attack Church officials in his In Praise of Folly? O For spending money on lavish art O For not allowing clergy to marry O For supporting military campaigns O For selling pardons and...
-
Your company has just decided to purchase 50 percent of its inventory from China and purchases will be invoiced in Chinese yuan. What four processes do you need to consider in designing a foreign...
-
Why might changes in household decision-making cause a deviation in GDP from trend?
-
Using Program16.m(Houbolt method), solve Problem 11.19. Data From Problem 11.19:- Using the central difference method, find the response of the system shown in Fig. 11.2 when \(F_{1}(t)=10 \sin 5 t\)...
-
Audrinas Fleet Feet, Inc., produces dance shoes for stores all over the world. While the pairs of shoes are boxed individually, they are crated and shipped in batches. The shipping department records...
-
Analyze the Business Strategy for the company "Uber" that is similar to your "Green Drive" application. Provide a report by analyzing the strategies that uber is using/used. Using Porter's Five...
-
You have 100 grams of water at 25C in a container that holds exactly 200 ml. What mass of methanol do you need to add to the system such that the container is filled without overflowing? See the...
-
This problem involves the same compound that was examined in Problems 6-14 through 6-17, which in the vapor phase was described by the EOS: A. The fugacity in the vapor phase at T = 50C and P = 0.1...
-
Shown below are the histogram and summary statistics for the number of camp sites at public parks in Vermont. a) Which statistics would you use to identify the center and spread of this distribution?...
-
An ambulance generally transports between one and eight patients a day. If the ambulance crew reports no particular pattern within that range, what is the expected value of the daily number of...
-
There is a 60% chance of a loss of $100, a 30% chance of sales of $100, and a 10% chance of sales of $200. What is the expected value of sales?
-
A processer has a 10% probability of freezing, at which point an identical backup processor is activated. What is the probability they both freeze?
-
In general, how is the equivalent stiffness of a combination of springs calculated?
-
A component has a reliability of 98% and is backed up by a redundant component with the same probability. What is their combined reliability?
-
How does the translation of depreciation and amortization differ under the current-rate method as opposed to the temporal method?
-
Consider model (9.18). What is the effect on the model parameter estimates, their standard errors, and the goodness-of-fit statistics when (a) The times at risk are doubled, but the numbers of deaths...
-
The useful life of a machine bearing depends on its operating temperature, as the following data show. Obtain a functional description of these data. Plot the function and the data on the same plot....
-
A certain electric circuit has a resistor and a capacitor. The capacitor is initially charged to 100 V. When the power supply is detached, the capacitor voltage decays with time, as the following...
-
The distance a spring stretches from its free length is a function of how much tension force is applied to it. The following table gives the spring length y that was produced in a particular spring...
-
Midnight Sun Apparel Company uses normal costing, and manufacturing overhead is applied to work-in-process on the basis of machine hours. On January 1 of the current year, there were no balances in...
-
what methodologies can enterprises employ to meticulously orchestrate transformative processes, leveraging innovative technologies, cultivating interdisciplinary synergies, and ameliorating...
-
Jack, age 3 0 and married with no dependents, is a self - employed individual. For 2 0 2 3 , his self - employed business sustained a net loss from operations of $ 1 0 , 0 0 0 from operations . The...

Study smarter with the SolutionInn App


