Consider the novel device for oxidative treatment of wastewater shown in the figure (right column). In this
Question:
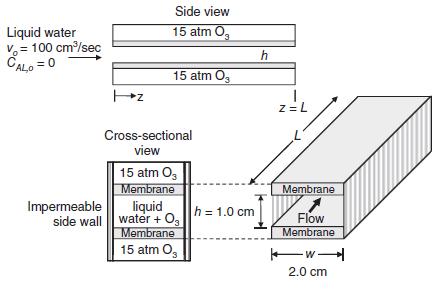
Consider the novel device for oxidative treatment of wastewater shown in the figure (right column). In this device, O3 will serve as the oxidant source, which must be carefully dosed into the liquid. The device consists of a vertical slit, where the walls of the slit consist of polymeric membranes that are selectively permeable to ozone gas (O3). As liquid water flows through the slit, O3 dissolves into the liquid. Assuming that the membrane offers no substantial resistance to O3 transfer, the concentration of water at the inner surface of the membrane in the liquid is adequately described by Henry’s law. For example, x*A = pA/H, where pA is the partial pressure of O3 on the gas side of the membrane, and xA is the mole fraction of dissolved O3 in the liquid right at the inner liquid surface side of the membrane, and the Henry’s law constant is H = 3760 atm at 20 C. At the present conditions of operation, a single slit has a slit opening h = 1.0 cm, and slit width w = 2.0 cm. The volumetric flow rate of liquid water into a single slit is 100 cm3/s. The inlet water contains no dissolved O3. The process is dilute, with total molar concentration of the liquid equal to 0.056 gmole/cm3. At 20 C, the diffusion coefficient of dissolved O3 (solute A) in liquid water (solvent B) is DAB 1.74 10 5 cm2/s, and the kinematic viscosity of liquid water is 0.010 cm2/s. The partial pressure of pure O3 gas on the gas side of the membrane is maintained constant at 15.0 atm.
a. The mass-transfer coefficient associated with “flow through a slit” can be converted to “flow through a tube” by simply considering an equivalent hydraulic diameter d 2h/π. What is the convective mass-transfer coefficient kL for O3 transfer for the water inside the slit?
b. Develop a material balance model, in final integrated form, to predict the dissolved concentration of O3 at the outlet (z = L). State your primary assumptions, and clearly show the development of your differential model before you perform the final integration. The material balance model must reflect the geometry of the process. What is the required length L for cAL = 2.0 gmole/m3?
c. If PA for O3 on the gas side of the membrane is increased from 15 to 30 atm, what is the new required length L to achieve a desired cAL of 2.0 gmole/m3?
d. Modify the process to include a UV light source shining into the slit through the semitransparent membrane material, and then repeat part (b). The UV light promotes a homogeneous first-order degradation reaction of O3 dissolved in solution, with the rate equation given by RA = –k1 cAL. At the conditions of illumination, k1 = 0.00050 s–1. Plot out cAL vs. z until the curve is flat, and compare this plot to a plot of cAL vs. z for the model in part (b).
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781119723547
7th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





