The photocatalytic tubular reactor shown in the figure (next page) is used to decompose organic contaminants in
Question:
The photocatalytic tubular reactor shown in the figure (next page) is used to decompose organic contaminants in liquid waste water. The inter walls of the 1.5 cm inner diameter glass tube are lined with a thin, nonporous, transparent layer of nanocrystalline titanium dioxide (TiO2), which catalyzes the oxidation of dissolved organic chemicals to carbon dioxide and water, using sunlight to drive this photocatalytic process. In the present process, waste water containing 10 g/m3 of benzene (solute A) dissolved in water enters the tube at 313 K, and it is necessary to have a Reynolds number (Re) of 5000 for flow through the tube. The steady state process temperature is 313 K. For purposes of analysis, assume the consumption of species A at the catalyst surface is so rapid that the effective concentration of species A at the catalyst surface is equal to zero (cAL,s ≈ 0). Potentially useful information at 313 K: molecular diffusion coefficient of benzene (C6H6, species A) in liquid water, DAB = 1.45 x 10–5 cm2/s, MA = 78 g/gmole, μB = 0.00658 g/cm-s (0.658 cP), ρB = 0.993 g/cm3.
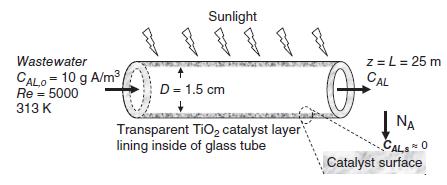
a. Identify the source and sink for mass transfer of benzene.
b. Is the photocatalytic reaction is best represented as a homogeneous reaction within the control volume for mass transfer or a heterogeneous reaction at a boundary surface? Justify your response.
c. Estimate the convective mass transfer coefficient of benzene in water flowing through the tube, kL.
d. Develop a model, in final integrated form, to estimate the concentration of species A exiting the tube. State three primary assumptions of your model. The model must at least contain the following variables: cAL,o, cAL, kL, L, v∞, D.
e. If the tube length (L) is 25.0 m, what is the concentration of benzene in the wastewater exiting the tube, i.e. cAL at z = L?
f. Consider now that the surface reaction is slow enough so that the benzene concentration on the catalyst is cAL,s > 0, and the surface reaction is described by a first-order process with ks = 0.0010 cm/s. Estimate the new outlet concentration of benzene.
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781119723547
7th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





