Ammonia vapor is compressed inside a cylinder by an external force acting on the piston. The ammonia
Question:
Ammonia vapor is compressed inside a cylinder by an external force acting on the piston. The ammonia is initially at 30◦C, 500 kPa, and the final pressure is 1400 kPa. The following data have been measured for the process: 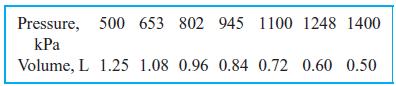
Determine the work done by the ammonia by summing the area below the P–V process curve. As you plot it, P is the height and the change in volume is the base of a number of rectangles.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:





