A 10.0 g sample of liquid water is sealed in a 1515 mL flask and allowed to
Question:
A 10.0 g sample of liquid water is sealed in a 1515 mL flask and allowed to come to equilibrium with its vapor at 27 °C. What is the mass of H2O(g) present when equilibrium is established? Use vapor pressure data from Table 12.5.
Table 12.5
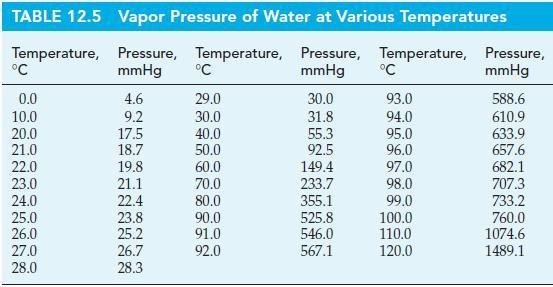
Transcribed Image Text:
TABLE 12.5 Temperature, °C 0.0 10.0 20.0 21.0 22.0 23.0 24.0 25.0 26.0 27.0 28.0 Vapor Pressure of Water at Various Temperatures Pressure, Temperature, Pressure, Temperature, mmHg mmHg 4.6 9.2 17.5 18.7 19.8 21.1 22.4 23.8 25.2 26.7 28.3 °C 29.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0 91.0 92.0 30.0 31.8 55.3 92.5 149.4 233.7 355.1 525.8 546.0 567.1 °C 93.0 94.0 95.0 96.0 97.0 98.0 99.0 100.0 110.0 120.0 Pressure, mmHg 588.6 610.9 633.9 657.6 682.1 707.3 733.2 760.0 1074.6 1489.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Find the vapor pressure of water at 27 C From Table 125 the vapor press...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
What will be the profit percentage after selling an article at certain price if there is a loss of 40 percent when the same article is sold at 2/5 of the earlier selling price?
-
Find given En+1 10 = 3 n = and = 4.
-
Which of the following are the government inspectors whose mission is to reduce Medicare improper payments through the detection and collection of overpayments, identification of underpayments, and...
-
In 1976, Mohamed EI-Iladad earned an undergraduate accounting degree in his native Egypt. Before he began his accounting career, El-Hadad completed his compulsory service in the Egyptian military...
-
Plaintiff brings this cause of action against a manufacturer for the loss of one leg below the hip. The leg was lost when caught in the gears of a screw auger machine sold and installed by the...
-
If there are conflicting priorities and disagreements among members of the IT steering committee, how might they be resolved?
-
Discuss the effects of internationalisation on pricing.
-
Post-Balance-Sheet Events Keystone Corporation issued its financial statements for the year ended December 31, 2010, on March 10, 2011. The following events took place early in 2011. (a) On January...
-
As a leader, how will you influence change in your professional specialty area? Identify two strengths you have that will promote strong leadership. Identify two areas of improvement to your skill in...
-
Some vapor pressure data for Freon-12, CCl 2 F 2 , once a common refrigerant, are -12.2 C, 2.0 atm; 16.1 C, 5.0 atm; 42.4 C, 10.0 atm; 74.0 C, 20.0 atm. Also, bp = -29.8 C, T c = 111.5 C, P c = 39.6...
-
A 7.53 L sample of N 2 (g) at 742 mmHg and 45.0 C is bubbled through CCl 4 (l) at 45.0 C. Assuming the gas becomes saturated with CCl 4 (g) what is the volume of the resulting gaseous mixture if the...
-
The following ratios were computed from the 2019 financial statements of Nordstrom and Macy's. Explain why Nordstrom has a higher ROE and a higher ROA. Nordstrom Macy's ROE 60.9% 18.21% ROA 7.00%...
-
What are two key points required to prepare for the interview process? Explain and provide example.
-
Current Attempt in Progress Sheridan Corporation issued 380 shares of $12 par value common stock for $6,840. Prepare Sheridan's journal entry. (List all debit entries before credit entries. Credit...
-
Identify and describe the basic and precision cuts used in commercial kitchen?
-
! Required information [The following information applies to the questions displayed below.] Stark company has the following adjusted accounts and normal balances at its December 31 year-end. Notes...
-
The Massoud Consulting Group reported net income of $1,366,000 for its fiscal year ended December 31, 2024. In addition, during the year the company experienced a positive foreign currency...
-
Suppose we observe the following rates: 1R1 = 10%, 1R2 = 14% and E(2r1) = 10%. If the liquidity premium theory of the term structure of interest rates holds, what is the liquidity premium for year 2?
-
CdF2 (s) Cd+ (aq) + 2 F- (aq) 1. A saturated solution of CdF2 is prepared. The equilibrium in the solution is represented above. In the solution [Cd+] eq = 0.0585 M and [F-] eq = 0.117 M. a....
-
Dividend Growth Model Based on the dividend growth model, what are the two components of the total return on a share of stock? Which do you think is typically larger?
-
Growth Rate In the context of the dividend growth model, is it true that the growth rate in dividends and the growth rate in the price of the stock are identical?
-
Voting Rights When it comes to voting in elections, what are the differences between US political democracy and U.S. corporate democracy?
-
Define two classes. The first class contains a method int calculateMultiplication(int, int) that takes 2 integer arguments and returns the multiplication of them. The second class contains the main...
-
What are the key architectural differences between monolithic kernels and microkernels, and how do these differences impact the performance, security, and maintainability of an operating system ?
-
List four guidelines for an applicant to start a job interview off on the right foot

Study smarter with the SolutionInn App


