A 625 mL sample of an aqueous solution containing 0.275 mol propionic acid, CH 3 CH 2
Question:
A 625 mL sample of an aqueous solution containing 0.275 mol propionic acid, CH3CH2CO2H, has [H3O+] = 0.00239 M. What is the value of Ka for propionic acid?
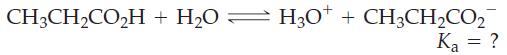
Transcribed Image Text:
CH3CH₂CO₂H + H₂0 H3O+ + CH3CH₂CO₂ Ka = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Write the equilibrium expression for the dissociation of propionic acid The equilibrium expre...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
Adelyn is in a financial dispute with her creditor. She wants to declare bankruptcy because she is finding herself unable to meet the requirements of paying off her debt. Which court that A would...
-
What is the pH of 250.0 mL of an aqueous solution containing 0.616 g of the strong acid trifluoromethane sulfonic acid (CF3SO3H)?
-
Craig Industries was in the business of manufacturing charcoal. Craig, the corporation's president, contracted in the name of the corporation to sell the company's plants to Husky Industries. Craig...
-
Kansas City Castings (KCC) is attempting to obtain the maximum loan possible using accounts receivable as collateral. The firm extends net-30-day credit. The amounts that are owed KCC by its 12...
-
You have been hired by the management of Alden, Inc. to review its control procedures for the purchase, receipt, storage, and issuance of raw materials. You prepared the following comments, which...
-
Gilead is a large drug producer, with a majority of its prescription drug product sales occurring in the United States. Gilead produces anti-HIV drug therapies, including the drugs Atripla, Truvada,...
-
For this exercise, your client, Bright IDEAs Inc., has provided you with data for two related files, a listing of sales invoices, and a listing of customers with credit limits. To test whether credit...
-
Adama Is A French Manufacturer Of Photovoltaic Panels. The Company Has A Production Plant In Rennes, Which Supplies Four Warehouses Located In Angers, Bourges, Clermont-Ferrand And Montauban. The...
-
Fluoroacetic acid occurs in gifblaar, one of the most poisonous of all plants. A 0.318 M solution of the acid is found to have a pH = 1.56. Calculate K a of fluoroacetic acid. CHFCOOH(aq) + HO = H3O+...
-
What are the [H 3 O + ] and pH of 0.143 M HNO 2 ?
-
Newmarge Products Inc. is evaluating a new design for one of its manufacturing processes. The new design will eliminate the production of a toxic solid residue. The initial cost of the system is...
-
What is the length of a side of the small inner square?
-
If f(x)=tan(In3x), find f'(x)
-
Evaluate 3 1-x dx. x= sin 0
-
Let f (x) = x + 13x +36 and g(x) = x +9 Find (fg) (x)
-
Consider a loan of $225,000 at nominal interest rate of 6.5% for 15 years. How much of the payment during the first year goes towards interest? Show calculation
-
Two reading selections from Voltaires Candide. The story begins by acknowledging the foolishness of the superstitious traditions held by the people in the city of Lisbon but ends by confirming...
-
How has the too-big-to-fail policy been limited in the FDICIA legislation? How might limiting the too-big-to-fail policy help reduce the risk of a future banking crisis?
-
What is the nature of the balance in the prepaid insurance account at the end of the accounting period? (a) Before adjustment? (b) After adjustment?
-
On July 1 of the current year, a business paid the July rent on the building that it occupies. (a) Do the rights acquired at July 1 represent an asset or an expense? (b) What is the justification for...
-
(a) Explain the purpose of the two accounts: Depreciation Expense and Accumulated Depreciation. (b) What is the normal balance of each account? (c) Is it customary for the balances of the two...
-
Considering the Venn Diagram below, where regions have been shaded in different colors and labeled with numbers, in which numbered region would the student described in each part belong? A B 3 5 8 4...
-
a) Find x such that the vectors (3,2,x) and (2x,4,x) are orthogonal. b) Find the direction cosines and direction angles of the vector a=3i-2j-3k. Give the direction angles correct to the nearest...
-
Suppose you plan to select 100 random samples of size N = 25 from a population of intelligence test scores with p = 100 and = 15. a. What proportion of the sample means do you expect to be between 97...

Study smarter with the SolutionInn App


