A classic experiment in equilibrium studies dating from 1862 involved the reaction in solution of ethanol (C
Question:
A classic experiment in equilibrium studies dating from 1862 involved the reaction in solution of ethanol (C2H5OH) and acetic acid (CH3COOH) to produce ethyl acetate and water.
![]()
The reaction can be followed by analyzing the equilibrium mixture for its acetic acid content.
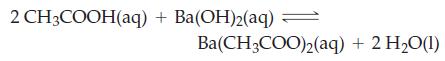
In one experiment, a mixture of 1.000 mol acetic acid and 0.5000 mol ethanol is brought to equilibrium. A sample containing exactly one-hundredth of the equilibrium mixture requires 28.85 mL 0.1000 M Ba(OH)2 for its titration. Calculate the equilibrium constant, Kc, for the ethanol-acetic acid reaction based on this experiment.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





