A description of bonding in XeF 2 based on the valence bond model requires the 5d orbitals
Question:
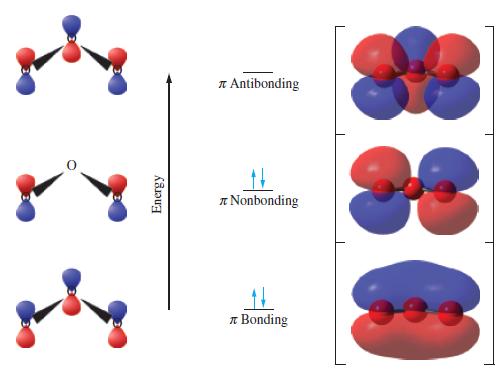
A description of bonding in XeF2 based on the valence bond model requires the 5d orbitals of Xe. A more satisfactory description uses a molecular orbital approach involving three-center bonds. Assume that bonding involves the 5pz orbital of Xe and the 2pz orbitals of the two F atoms. These three atomic orbitals combine to give three molecular orbitals: one bonding, one nonbonding, and one antibonding. Recall that for bonding to occur, atomic orbitals with the same phase must overlap to form bonding molecular orbitals.
(a) Construct diagrams similar to Figure 11-33 to indicate the overlap of the three atomic orbitals in forming the three molecular orbitals. Assume that the order of energy of the molecular orbitals is bonding MO
(b) Sketch a molecular orbital energy-level diagram, and assign the appropriate number of electrons from fluorine and xenon to the molecular orbitals. What is the bond order?
(c) With the aid of VSEPR theory, show that this molecular orbital description based on three-center bonds works well for XeF4 but not for XeF6.
Figure 11-33
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





