(A) In a proposed two-step mechanism for the reaction CO(g) + NO 2 (g) CO 2...
Question:
(A) In a proposed two-step mechanism for the reaction CO(g) + NO2(g) → CO2(g) + NO(g), the second, fast step is NO3(g) + CO(g) → NO2(g) + CO2(g). What must be the slow step? What would you expect the rate law of the reaction to be? Explain.
(B) Show that the proposed mechanism for the reaction 2 NO2(g) + F2(g) → 2 NO2F(g) is plausible. The rate law is rate = k[NO2][F2].
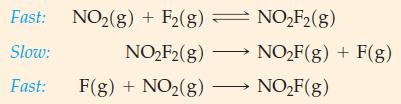
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





