A mixture of 1.00 mol NaHCO 3 (s) and 1.00 mol Na 2 CO 3 (s) is
Question:
A mixture of 1.00 mol NaHCO3(s) and 1.00 mol Na2CO3(s) is introduced into a 2.50 L flask in which the partial pressure of CO2 is 2.10 atm and that of H2O(g) is 715 mmHg. When equilibrium is established at 100 °C, will the partial pressures of CO2(g) and H2O(g) be greater or less than their initial partial pressures? Explain.
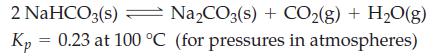
Transcribed Image Text:
2 NaHCO3(s) → Na₂CO3(s) + CO₂(g) + H₂O(g) 0.23 at 100 °C (for pressures in atmospheres) Kp
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine whether the partial pressures of CO2g and H2Og will be greater or less than their initi...View the full answer

Answered By

David Muchemi
I am a professional academic writer with considerable experience in writing business and economic related papers. I have been writing for my clients who reach out to me personally after being recommended to me by satisfied clients.
I have the English language prowess, no grammatical and spelling errors can be found in my work. I double-check for such mistakes before submitting my papers.
I deliver finished work within the stipulated time and without fail. I am a good researcher on any topic especially those perceived to be tough.
I am ready to work on your papers and ensure you receive the highest quality you are looking for. Please hire me to offer my readily available quality service.
Best regards,
4.60+
27+ Reviews
61+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A 25-g block of iron at 175C is dropped into a liter of water in an insulated flask at 20C and 1 atm. The specific enthalpy of iron is given by the expression H(J/g)= 17.3T(C). (a) What reference...
-
In chemical vapor deposition (CVD), a semiconducting or insulating solid material is formed in a reaction between a gaseous species and a species adsorbed on the surface of silicon wafers (disks...
-
A mixture of 1 mol of H2O, 2 mol of O2, and 5 mol of N2 is heated to 2200 K at a pressure of 5 atm. Assuming the equilibrium mixture consists of H2O, O2, N2, and H2, determine the equilibrium...
-
Logical fallacies are frequently used in arguments and have an intuitive appeal that makes them effective for politicians to use. Using your understanding of fallacies, you will listen to and take...
-
Southland Industries has $60,000 of 16% (annual interest) bonds outstanding, 1,500 shares of preferred stock paying an annual dividend of $5 per share, and 4,000 shares of common stock outstanding....
-
Tyler Pahl recently received the following information related to Pahl Companys December 31, 2014, balance sheet. Inventory ................$ 4,100 Cash .................. 3,900 Equipment...
-
Motion Auto would like to assign the oldest costs of inventory items to its ending inventory. Which inventory costing method should Motion Auto choose?
-
Givoly Inc. uses a periodic inventory system. At the end of the annual accounting period, December 31 of the current year, the accounting records provided the following information for product 2:...
-
The process flow below shows four steps involved in admitting patients into a specialty clinic. Step 3 has two people assigned, while all other steps have a single person assigned. S1 1 person S2 1...
-
Cadmium metal is added to 0.350 L of an aqueous solution in which [Cr 3+ ] = 1.00 M. What are the concentrations of the different ionic species at equilibrium? What is the minimum mass of cadmium...
-
Formamide, used in the manufacture of pharmaceuticals, dyes, and agricultural chemicals, decomposes at high temperatures. If 0.186 mol HCONH 2 (g) dissociates in a 2.16 L flask at 400 K, what will be...
-
Do you think it is right or fair for some countries to have strong anti-bribery laws while other countries accept bribery as a way of doing business?
-
Given: pH = -log [H*] If the pH is 6.5 what is [H+]? Give 3 significant figures.
-
You are in your junior semester in the Health Services Administration program. You've been in college for more than two years now. Think about the skills you have developed in other courses that...
-
Date March 1 March 5 March 9 March 18 March 25 March 29 Activities Beginning inventory Purchase Sales Purchase Purchase Sales Totals Units Acquired at Cost $52.60 per unit 180 units 265 units 125...
-
1. Advocate Aurora Sheboygan Memorial Hospital health care product or service to be marketed in your community. 2. Conduct appropriate market research in your community to determine the demographics...
-
A stock price is currently trading at $100. Over the next two 6 month periods it will be with up 10% or down 10%. The risk-free interest rate is 8% per annum with continous compunding. What is the...
-
In an effort to develop more accurate product costs, Bell Corporation decided to adopt an activity-based costing (ABC) system. However, much to managements surprise, the product costs determined...
-
What are the principal differences among asset liquidity management, liability management, and balanced liquidity management?
-
The following are inputs and outputs to the copying process of a copy shop: Percent jobs done on time Number of times paper supply runs out Number of pages copied per hour Number of employee errors...
-
Bavarian Chocolate Company produces chocolate bars. The primary materials used in producing chocolate bars are cocoa, sugar, and milk. The standard costs for a batch of chocolate (5,000 bars) are as...
-
Hickory Furniture Company manufactures unfinished oak furniture. Hickory uses a standard cost system. The direct labor, direct materials, and factory overhead standards for an unfinished dining room...
-
CU, Incorporated (CUI), produces copper contacts that it uses in switches and relays. CUI needs to determine the order quantity, Q, to meet the annual demand at the lowest cost. The price of copper...
-
Aulman Inc. has a number of divisions including a Furniture Division and a Motel Division. The Motel Division owns and operates a line of budget motels located along major highways. Each year, the...
-
The operations vice president of Home Bank has been interested in investigating the efficiency of the bank's operations. She has been particularly concerned about the costs of handling routine...

Study smarter with the SolutionInn App


