A saturated aqueous solution of onitrophenol, HOC 6 H 4 NO 2 , has pH = 4.53.
Question:
A saturated aqueous solution of o–nitrophenol, HOC6H4NO2, has pH = 4.53. What is the solubility of o-nitrophenol in water, in grams per liter?
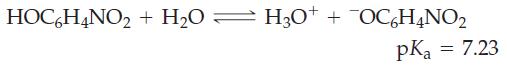
Transcribed Image Text:
HOC6H4NO₂ + H₂O = H3O+ H3O+ + OC6H4NO₂ pKa = 7.23
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To calculate the solubility of onitrophenol in waterwe can use the following equation pH ...View the full answer

Answered By

Sonal Sharma
I have taught chemistry in school as well as college students. I have got the opportunity to deal with their problems in chemistry through interact with them. I am sure once you enjoying the subject you definitely overcome with your subject difficulties .
0.00
0 Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A saturated aqueous solution of Ca(OH) 2 has a pH of 12.35. What is the solubility ofCa(OH) 2 , expressed in milligrams per 100 mL of solution?
-
A silver rod and a SHE are dipped into a saturated aqueous solution of silver oxalate, Ag2C2O4, at 25C. The measured potential difference between the rod and the SHE is 0.589 V, the rod being...
-
a. Calculate the solubility product of the following solutions: i. A saturated aqueous solution of cadmium sulfide, CdS (solubility = 1.46 10 11 mol dm 3 ) ii. A saturated aqueous solution of...
-
Santa's Helpers Ltd estimates its income taxes at 35% of pre-tax income. For the quarter ended September 30, pre-tax income was $200,000. Prepare the journal entry to record the estimated income...
-
What is leasing? Define, compare, and contrast operating leases and financial (or capital) leases. How does the Financial Accounting Standards Boards Statement No. 13 define a financial (or capital)...
-
Listed below are several types of accounting data that might be coded. For each data item, recommend a type of code (mnemonic, sequence, block, or group) and support your choice. a. Employee...
-
How might a hacker access and manipulate a digital device for illegal purposes? Are the Internet of Things (IoT) devices at risk for hacker access and manipulation?
-
The accountant of Weatherspoon Shoe Co. has compiled the following information from the companys records as a basis for an income statement for the year ended December 31, 2012. Rent revenue $ 29,000...
-
What is Martin Gardner's argument for the objectivist view of art? Do you agree? Why or why not? Use Vaughns textbook to help you explain Gardners theory and its strengths and weaknesses. Choose an...
-
A particular vinegar is found to contain 5.7% acetic acid, CH 3 COOH, by mass. What mass of this vinegar should be diluted with water to produce 0.750 L of a solution with pH = 4.52?
-
What are [H 3 O + ], [OH - ], pH, and pOH of 0.386 M CH 3 NH 2 ?
-
Add a condition to the deposit method of the BankAccount class in Section 22.5, restricting deposits to $100,000 (the insurance limit of the U.S. government). The method should block until sufficient...
-
Pinder Co . , Ltd . is considering building a solar power plant. The solar power plant will cost $ 1 0 million and will take one year to build. If the demand for electricity is large, solar power...
-
Question 38 (1 point) According to the Efficiency Wage Theory, giving efficiency wages to workers, may have the following impact: a) decreases the quality of the firm's workforce b) decreases the...
-
2019 Tax Rates, Single Individual Taxable Income Tax Payable $ 0 - $ 9,700 10% of TI $ 9,700 - $ 39,475 $ 970.00 + 12% of (TI - 9,700 ) $ 39,475 - $ 84,200 $ 4,543.00 + 22% of (TI - 39,475 ) $ 84,200...
-
Create an economic story that takes a fictional country through all the stages in the economic cycle. Include all needed graphs and numbers | Make sure to include the following topics: 2.1 Supply and...
-
Solve 8 0 8x = x dx -
-
Suppose that $1 billion of pass-throughs is used to create a CMO structure with a PAC bond with a par value of $700 million and a support bond with a par value of $300 million. Answer the question...
-
9.Consider the reaction 3NO2(g)+H2O=2HNO3(aq)+NO(g) where Delta H=-137 kJ.How many kilojoules are released when 92.3g of NO2 reacts?
-
The balance sheet for Bearing Industries Inc. at the end of the current fiscal year indicated the following: Bonds payable, 10% (issued in 2000 due in 2020) $4,000,000 Preferred $5 stock, $100 par...
-
The following information was taken from the financial statements of Finn Resources Inc. for December 31 of the current fiscal year: The net income was $600,000 and the declared dividends on the...
-
The table below shows the stock price, earnings per share, and dividends per share for three companies as of October 2007: (a) Determine the price-earnings ratio and dividend yield for the three...
-
Q2. A body of mass m is thrown straight up with velocity vo. Find the maximum height the body will go to if the air drag equals kv, where k is a constant and v is the velocity of the body.
-
What if: Assume Bill decides to purchase Intel stock for $50,000 and hold the shares for five years. If the Intel stock grows at a constant 8 percent before-tax rate and does not pay any dividends,...
-
Part A If you look at yourself in a shiny Christmas tree ball with a diameter of 9.4 cm when your face is 28.0 cm away from it, where is your image? Express your answer using two significant figures....

Study smarter with the SolutionInn App


