A solution is prepared having these initial concentrations: [Fe 3+ ] = [Hg 2 2+ ] =
Question:
A solution is prepared having these initial concentrations: [Fe3+] = [Hg22+] = 0.5000 M; [Fe2+] = [Hg2+] = 0.03000 M. The following reaction occurs among the ions at 25 °C.
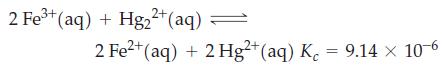
What will be the ion concentrations at equilibrium?
Transcribed Image Text:
2 Fe³+ (aq) + Hg2²+ (aq) 2 Fe²+ (aq) + 2 Hg2+ (aq) K = 9.14 x 10-6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Setting up the problem The balanced chemical equation for the reaction is 2 Fe3 aq Hg22 aq 2 Fe2 aq ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A solution is prepared from 0.150 mol of formic acid and enough water to make 0.425 L of solution. a. Determine the concentrations of H3O+ and HCOO in this solution. b. Determine the H3O+...
-
The following reaction occurs by a general-acid-catalyzed mechanism: Propose a mechanism for this reaction. CH2 CH3 HB+
-
The following reaction occurs by a mechanism involving general-base catalysis: Propose a mechanism for this reaction. base + CH,CH20H CH2OH
-
The annual revenues associated with several large apartment complexes are $300, $450, $425, $50, $75, and $150 for years 0, 1, 2, 3, 4, and 5, respectively. Determine the net cash flow and whether...
-
Security Data Company has outstanding 50,000 shares of common stock currently selling at $40 per share. The firm most recently had earnings available for common stockholders of $120,000, but it has...
-
Visit the Web site of an online catalog vendor similar to RMO (such as www.llbean.com) or an online vendor of computers and related merchandise (such as www.cdw.com). Browse the online catalog and...
-
PWC Corp. accounting records include the following items, listed in no particular order, at December 31, 2008 : Prepare PWC's income statement for the year ended December 31, 2008. Omit earnings per...
-
Assume a Gold Medal Sports outlet store began August 2012 with 44 pairs of running shoes that cost the store $33 each. The sale price of these shoes was $61. During August, the store completed these...
-
Problem 5 a. Control procedures implemented during June have reduced the actual food cost per- centage by 0.5 percent and the actual beverage cost percentage by 1.8 percent from the May levels...
-
Refer to the Integrative Example. A gaseous mixture is prepared containing 0.100 mol each of CH 4 (g), H 2 O(g), CO 2 (g), and H 2 (g) in a 5.00 L flask. Then the mixture is allowed to come to...
-
For the synthesis of ammonia at 500 K, N 2 (g) + 3 H 2 (g) 2 NH 3 (g), Kp = 9.06 x 10 -2 when the pressures are expressed in atmospheres. Assume that N 2 and H 2 are mixed in the mole ratio 1 : 3...
-
Discuss in detail Network LAN Design with VoIP, and Wireless Services. Address the following criteria in the light of Network LAN Design with VoIP, and Wireless Services Equipment List Hierarchical...
-
13. During a load rejection in the power plant illustrated in the figure below. The elevation in the surge tank varied sinusoidally with a maximum rate of change of 9.55 m/s. ho h h L h3 The chief...
-
Examine the role of Global Supply Chains and the related risks and opportunities in context to international business. Can residents live and work at the same place a tourist seeks enrichment,...
-
A positive charge, Q = +3.0 nC is placed at y = +5.0 cm and a negative charge Q = -3.0 nC is placed at y = -5.0 cm as shown in the figure. A third positive charge, q = +0.01 nC is placed at the...
-
Henrich is a single taxpayer. In 2023, his taxable income is $535,500. What are his income tax and net investment income tax liability in each of the following alternative scenarios? Use Tax Rate...
-
The path of a pumpkin launched from a compressed air cannon is modeled by f(x) - (a) How high (in ft) is the pumpkin when it is launched? (b) What is the maximum height (in ft) of the pumpkin?...
-
Business managers frequently operate in a world where data are not readily available. Two independent situations follow: Required A. Based on the information given above, reconstruct the accounting...
-
What are the key dimensions of critical thinking 2. Watch the NBC Learn video on Diet Scams. What types of claims are made in this video Are they valid Elaborate on your responses. Discuss this video...
-
An employee earns $40 per hour and 1.75 times that rate for all hours in excess of 40 hours per week. Assume that the employee worked 60 hours during the week, and that the gross pay prior to the...
-
Reaves Professional Services has three employees?a consultant, a computer programmer, and an administrator. The following payroll information is available for each employee: For the current pay...
-
In the following summary of data for a payroll period, some amounts have been intentionally omitted: (a) Calculate the amounts omitted in lines (1), (3), (8), and (12). (b) Journalize the entry to...
-
Given that bar (n) runs in (logn) time, write a recurrence relation for the time taken by function foo (): void foo(int n) { if (n < 1) return; for (int i = 0; i < n; ++i) for (int j=0; j < n; ++j)...
-
What is the Big- time complexity of foo() ? void foo(int n) { for (int i = 0; i < n; ++i) { bar(i); } baz(n); } void bar(int k) { for (int i=1; i < k/2; ++i) { baz(i); void baz(int x) { for (int i =...
-
One line divides the plane into two halves or regions, while two lines divide the plane into four regions. In this problem we study the number of regions in which n lines divide the plane. For...

Study smarter with the SolutionInn App


