(A) The solubility of CO 2 (g) in H 2 O at 25 C and under a...
Question:
(A) The solubility of CO2(g) in H2O at 25 °C and under a CO2(g) pressure of 1 atm is 1.45 g CO2/L. Air contains 0.037% CO2 by volume. Use this information, together with data from Table 16.5, to show that rainwater saturated with CO2 has a pH ≈ 5.6 (the normal pH for rainwater).
Table 16.5
(B) Often the following generalization applies to oxoacids with the formula EOm(OH)n (where E is the central atom): If m = 0, Ka ≈ 10-7; if m = 1, Ka ≈ 10-2; if m = 2, Ka is large; and if m = 3, Ka is very large.
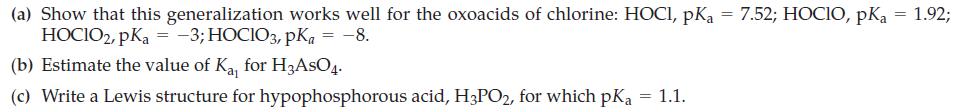
Transcribed Image Text:
TABLE 16.5 Ionization Constants of Some Polyprotic Acids Acid lonization Equilibria H₂S + H₂O HS + H₂O = Hydrosulfurica Carbonicb Citric Phosphoric Oxalic Sulfurouse Sulfuricd H₂CO3 + H₂O H₂O+ + HS¯ H₂O+ + S²- H₂SO4+H₂O HSO4+H₂O = HCO3+H₂0 H3C6H507 + H₂0 H₂CH²Ð‚¯ + H₂0 — HC H₂0₂2 + H₂0 — H₂PO4+H₂O H₂PO4+H₂O → HPO42- + H₂O = H₂C₂O4 + H₂0 — HC₂04 + H₂0 H₂SO3 + H₂O → H3O+ + HSO3- HSO3 + H₂0 H3O+ + SO3²- H₂0+ + HCO3- H3O+ + CO3²- H3O+ + H₂CH₂O, H3O+ + HC¿H₂0,²- K₁₂ H₂O+ + C₂H₂0,³- H₂O+ + H₂PO4¯ H3O+ + HPO4²- H3O+ + PO4³- H30+ + HC₂04¯ H30+ +C₂04²- lonization Constants, K K₁₁ = 1.0 × 10-7 = 1 × 10-19 Kaz Ka₁ H₂O+ + HSO4- H₂O+ + SO₂²- Kaz K₁₁ = 7.5 x 10-4 = 1.7 x 10-5 Ka Kal Kaz Kaj = 4.4 x 10-7 Kaz Kay Kaz Kaz Kay K₂ = 4.7 x 10-11 = 4.0 x 10-7 = 7.1 x 10-3 = 6.3 × 10-8 = 4.2 X 10-13 = 5.6 x 10-2 = 5.4 x 10-5 = 1.3 x 10-2 = 6.2 × 10-8 = very large = 1.1 x 10-2 pk pka₁ = 7.00 pka₂ = 19.0 pka₁ = 6.36 pka2 = 10.33 pK₁₁ = 3.12 PK₁₂ = 4.77 PK₁₂ = 6.40 PK₁₁ = 2.15 PK₁₂ = 7.20 pKaz = 12.38 pka = 1.25 PK₁₂ = 4.27 pk = 1.89 PK₁₂ = 7.21 PK₁₁ <0 pk az = 1.96 Acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
A The pH of rainwater saturated with CO2 can be estimated using the fact that CO2 dissolves in water ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The solubility of CO2 in water at 25C and 1 atm is 0.034 mol/L. What is its solubility under atmospheric conditions? (The partial pressure of CO2 in air is 0.0003 atm.) Assume that CO2 obeys Henry's...
-
In Exercise 112 in Chapter 5, the pressure of CO2 in a bottle of sparkling wine was calculated assuming that the CO2 was insoluble in water. This was an incorrect assumption. Redo this problem by...
-
Julio sold his corporation to a competitor, Exeter LLC, for $100,000,000. Julio incorporated his business 17 years ago by investing $500,000 plus his proprietary know-how. There have been no other...
-
Apple a Day, Inc., and Unforgettable Edibles, Inc., are fold catering businesses that operate in the same metropolitan area. Their customers include Fortune 500 companies, regional firms, and...
-
What causes bond prices to fluctuate?
-
Petitioner Salman was indicted for federal securities-fraud crimes for trading on inside information he received from a friend and relative-by-marriage, Michael Kara, who, in turn, had received the...
-
On May 1, Arnie Watson sent a memo to his boss, the director of project management, stating that the MX project would require thirteen weeks for completion according to the figure shown at the top of...
-
- Consider an asymmetric Cournot duopoly game, where the two firms have different costs of production. Firm 1 selects quantity 91 at a pro- duction cost of 2q. Firm 2 selects quantity 92 and pays the...
-
The structural formula shown is para-hydroxybenzoic acid, a weak diprotic acid used as a food preservative. Titration of 25.00 mL of a dilute aqueous solution of this acid requires 16.24 mL of 0.0200...
-
According to the Lewis theory, each of the following is an acidbase reaction. Which species is the acid and which is the base? BF4 (a) BF3 + F- (b) OH(aq) + CO(aq) HCO3(aq)
-
Gunny Stores, Inc., one of the nation's largest grocery retailers, reported the following information (adapted) in its comparative financial statements for the fiscal year ended January 31, 2015:...
-
Dryer Companys policy is to keep 25% of the next month's sales in ending inventory. If Dryer meets its ending inventory policy at the end of April and sales are expected to be 24,000 units in May and...
-
Why Is Leasing Profitable for Both the Lessor and Lessee? The symmetry between the lessee ' s problem and the lessor ' s problem suggests that if the lessee wants to lease, it will not be profi table...
-
On May 1, 2019, Red Hood Company issued P2,000,000, 10 years, 9% bonds at 105 including accrued interest. These bonds dated January 1, 2019. Interest is payable semi-annually on January 1 and July 1....
-
On Dec. 31, 2021, Green Inc. issued of its 8%, 10 year, P1,000 face value bonds with detachable stock warrants for P1,200,000. Each bond carried a detachable warrant for one share of Green's ordinary...
-
On April 1, 2020, Gretel Corp. issued at 99, P2,000 of its 8%, P1,000 bonds. The bonds are dated April 1, 2020, to mature on April 1, 2030 and pay interest on April 1 and November 1. Gretel paid...
-
The warrants of Microsystems Corporation allow the holder to buy a share of stock at $15.50 and are selling for $4.80. The stock price is currently $13.70. To what price must the stock go for the...
-
Calculate the change in entropy when 100 kJ of energy is transferred reversibly and isothermally as heat to a large block of copper at (i) 0 C, (ii) 50 C.
-
Dossett Company had the following transactions pertaining to stock investments. Feb. 1 Purchased 600 shares of Goetz common stock (2%) for $6,000 cash, plus brokerage fees of $200. July 1 Received...
-
Wyrick Inc. had the following transactions pertaining to investments in common stock . Jan. 1 Purchased 2,500 shares of Murphy Corporation common stock (5%) for $140,000 cash plus $2,100 brokers...
-
On February 1, Neil Company purchased 500 shares (2% ownership) of Young Company common stock for $30 per share plus brokerage fees of $400. On March 20, Neil Company sold 100 shares of Young stock...
-
Is -1 correct? sentix Sentiment-Bitcoins (in USD) 55000- sertix Sentiment Becoins Ht: 0.263 50000- 45000 40000- 35000 30000- 25000- 20000- 15000- 10000- Jan 21 Bitcoin (USD) 27429 -05 -0.4 Jan 22...
-
According to The Comic Book History of Comics, the direct market: Group of answer choices A. Made it easier for fans to find specific comic books B. Shifted the financial risk from publisher to...
-
What parts of Medicare are managed by Medicare-approved private insurance companies? Group of answer choices Part C and Part D Part B and Part D Part A and Part B Part A and Part C

Study smarter with the SolutionInn App


