(A) Use data from Table 19.1 to predict the probable products when Pt electrodes are used in...
Question:
(A) Use data from Table 19.1 to predict the probable products when Pt electrodes are used in the electrolysis of KI(aq).
Table 19.1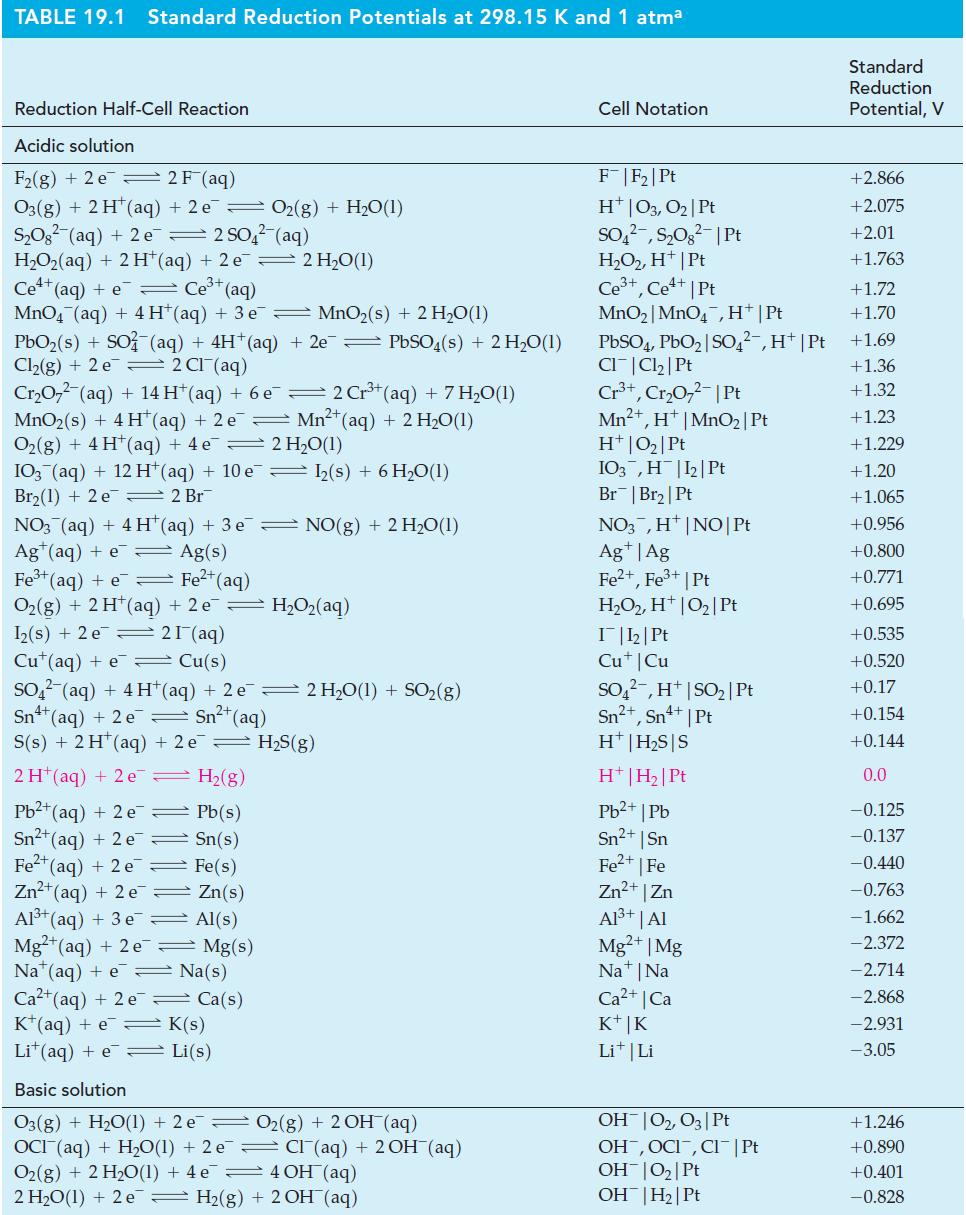
(B) In the electrolysis of AgNO3(aq), what are the expected electrolysis products if the anode is silver metal and the cathode is platinum?
Transcribed Image Text:
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma
Reduction Half-Cell Reaction
Acidic solution
F₂(g) + 2 e 2 F (aq)
O3(g) + 2 H+ (aq) + 2 e O₂(g) + H₂O(1)
S₂O² (aq) + 2 e
2 SO4²(aq)
H,Oz(aq) + 2H*(aq) +2e <2H,O(1)
Ce+ (aq) + e Ce³+ (aq)
MnO₂ (aq) + 4H*(aq) + 3 e¯ ⇒ MnO₂(s) + 2 H₂O(1)
PbO₂ (s) + SO² (aq) + 4H+ (aq) + 2e¯ — PbSO4(s) + 2 H₂O(1)
Cl₂(g) + 2 e 2 Cl(aq)
Cr₂O7² (aq) + 14 H*(aq) + 6 e¯ — 2 Cr³+ (aq) + 7 H₂O(1)
MnO2 (s) + 4H+ (aq) + 2 e Mn²+ (aq) + 2 H₂O(1)
O₂(g) + 4H+ (aq) + 4 e
2 H₂O(1)
IO3(aq) + 12 H+ (aq) + 10 e
2 Br
Br₂(1) 2 e
NO3(aq) + 4H(aq) + 3 e¯¯ — NO(g) + 2 H₂O(1)
Ag¹(aq) + e
Ag(s)
Fe³+ (aq) + e
Fe²+ (aq)
Oz(g) +2H*(aq) +2e
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma
Reduction Half-Cell Reaction
Acidic solution
F₂(g) + 2 e 2 F (aq)
O3(g) + 2 H+ (aq) + 2 e O₂(g) + H₂O(1)
S₂O² (aq) + 2 e
2 SO4²(aq)
H,Oz(aq) + 2H*(aq) +2e <2H,O(1)
Ce+ (aq) + e Ce³+ (aq)
MnO₂ (aq) + 4H*(aq) + 3 e¯ ⇒ MnO₂(s) + 2 H₂O(1)
PbO₂ (s) + SO² (aq) + 4H+ (aq) + 2e¯ — PbSO4(s) + 2 H₂O(1)
Cl₂(g) + 2 e 2 Cl(aq)
Cr₂O7² (aq) + 14 H*(aq) + 6 e¯ — 2 Cr³+ (aq) + 7 H₂O(1)
MnO2 (s) + 4H+ (aq) + 2 e Mn²+ (aq) + 2 H₂O(1)
O₂(g) + 4H+ (aq) + 4 e
2 H₂O(1)
IO3(aq) + 12 H+ (aq) + 10 e
2 Br
Br₂(1) 2 e
NO3(aq) + 4H(aq) + 3 e¯¯ — NO(g) + 2 H₂O(1)
Ag¹(aq) + e
Ag(s)
Fe³+ (aq) + e
Fe²+ (aq)
Oz(g) +2H*(aq) +2e
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use data from Table 19.1, as necessary, to predict the probable products when Pt electrodes are used in the electrolysis of (a) CuCl 2 (aq); (b) Na 2 SO 4 (aq); (c) BaCl 2 (l); (d) KOH(aq). Table...
-
Use data from Table to estimate ÎH for the combustion of methane (CH4), as shown below: Table s | 14 39 95 45 72 1 1419 6847064968 77386 42222 34 985 0302 121 Si H C O 437 490 9 31222241122...
-
An n-degree polynomial p(x) is an equation of the form p(x) = [ax, i=0 where x is a real number and each a; is a constant. a. Describe a simple O(n) time method for computing p(x) for a particular...
-
Solve the compound linear inequality graphically. Write the solution set in set-builder or interval notation, and approximate endpoints to the nearest tenth whenever appropriate. 1.59.10.5x6.8
-
Selected accounts from the adjusted trial balance for Louise's Gourmet Shop as of March 31, 2011, the end of the current fiscal year. The merchandise inventory for Louise's Gourmet Shop was $38,200...
-
Kalogridis Corp. manufactures industrial dye. The company is preparing its 2011 master budget and has presented you with the following information: a. The projected December 31, 2010, balance sheet...
-
Brian Rafferty ingested finasteride, a drug prescribed to him to treat an enlarged prostate. Finasteride is a generic version of Proscar, a drug manufactured by Merck. Some time after he started to...
-
The following transactions pertain to Smith Training Company for 2016: Jan. 30 Established the business when it acquired $45,000 cash from the issue of common stock. Feb. 1 Paid rent for office space...
-
Which is an invalid access for the array? integer x integer array (3) numsList numsList [0] = 1 numsList [1] = 2 numsList[2] = 0 x = 3
-
(A) K sp for AgCl = 1.8 x 10 -10 . What would be the measured E cell for the voltaic cell in Example 19-11 if the contents of the anode half-cell were saturated AgCl(aq) and AgCl(s)? Example 19-11...
-
With the data given for reaction (19.23), calculate K sp for AgI. Reaction (19.23) AgI(s) Ag+ (aq) + (aq) Ksp = ?
-
Figure E3.3 shows a pulse function,u(t). (a) From the information shown in Fig. 3.3, calculate the pulse width, t w . (b) Express u(t) as the sum of simpler functions (some perhaps translated in...
-
Suppose the Modigliani-Miller assumptions hold, and we have no taxes. Cantrasus Inc. currently has no debt. The market value of its equity is $10,000,000. The beta of the equity is 1.5. Cantrasus...
-
why is understanding population growth contributing to climate change important? What can be done to resolve this issue if we understand the relationship?
-
Problem 5 [40 pts]: In this problem, there is a knowledge base KB: as well as a query Q: KB = (a b)^ (a>c) Q=(c b) Use three different techniques show that this claim C (propositional logic sentence)...
-
You are analyzing the cost of debt for a firm. You know that the firms 14-year maturity, 7.0 percent coupon bonds are selling at a price of $856.71. The bonds pay interest semiannually. If these...
-
Shelton Co. purchased a parcel of land six years ago for $872,500. At that time, the firm invested $144,000 in grading the site so that it would be usable. Since the firm wasn't ready to use the site...
-
According to a report by Scarborough Research, the average monthly household cellular phone bill is $73. Suppose local monthly household cell phone bills are normally distributed with a standard...
-
For each of the following reactions, express the equilibrium constant: a) H20 (I) H2 (g) + 02 (g) Ke = 1.0x107 b) Fe2 (g) 2F (g) Ke= 4.9 x 10-21 c) C (s) + O2 (g) d) H2 (g) + C2H4 (g) C2H6 (g) Ke =...
-
Bunge Corporation has purchased two securities for its portfolio. The first is a stock investment in Longley Corporation, one of its suppliers. Bunge purchased 10% of Longley with the intention of...
-
Bartlet Financial Services Company holds a large portfolio of debt and stock securities as an investment. The total fair value of the portfolio at December 31, 2010, is greater than total cost. Some...
-
The Securities and Exchange Commission (SEC) is the primary regulatory agency of U.S. financial markets. Its job is to ensure that the markets remain fair for all investors. The following SEC sites...
-
| Consider the density function (mass per unit area) d(x, y) =x^2y^2. Choose the shapes in figure 2 that can possess 5(x, y)as their density function. Justify your answers. Determine which one of...
-
11. At a particular moment, three charged particles are located as shown in figure 4. Each charge is a mulitple of q = 1 nC. Find the net field at the location < 2,0,0> cm. (Your answer should be...
-
Provide an analysis of your chosen article by applying (and citing) the frameworks, theories and concepts from cross-cultural management. You do not have to agree with the arguments in the articles...

Study smarter with the SolutionInn App


