(A) With as much detail as possible, describe the phase changes that would occur if a sample...
Question:
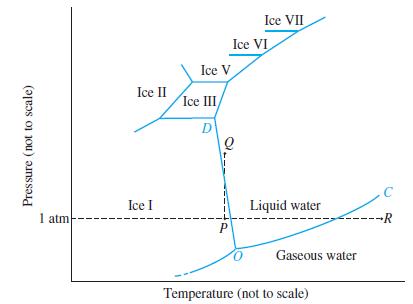
(A) With as much detail as possible, describe the phase changes that would occur if a sample of water represented by point R in Figure 12-30 were brought first to point P and then to point Q.
Figure 12-30
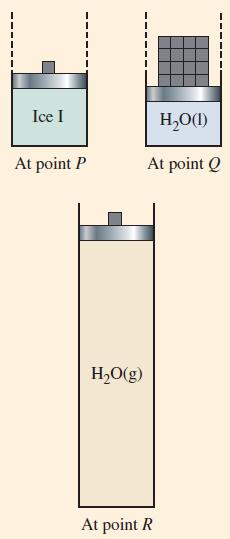
(B) Draw a sketch showing the condition prevailing along the line PR when 1.00 mol of water has been brought to the point where exactly one-half of it has vaporized. Compare this to the condition at point R in Figure 12-31, assuming that this is also based on 1.00 mol of water. For example, is the volume of the system the same as that in Figure 12-31? If not, is it larger or smaller, and by how much? Assume that the temperature at point R is the same as the critical temperature of water and that water vapor behaves as an ideal gas.
Figure 12-31
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




