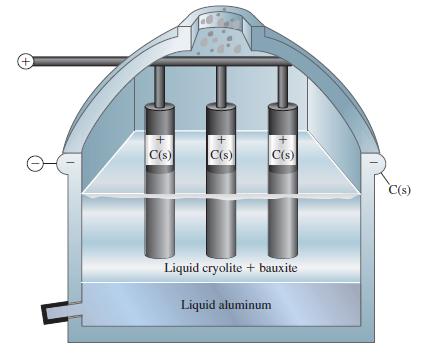
An aluminum production cell of the type pictured in Figure 21-24 operates at a current of 1.00
Question:
An aluminum production cell of the type pictured in Figure 21-24 operates at a current of 1.00 x 105 A and a voltage of 4.5 V. The cell is 38% efficient in using electrical energy to produce chemical change. (The rest of the electrical energy is dissipated as thermal energy in the cell.)
(a) What mass of Al can be produced by this cell in 8.00 h?
(b) If the electrical energy required to power this cell is produced by burning coal (85% C; heat of combustion of C = 32.8 kJ/g) in a power plant with 35% efficiency, what mass of coal must be burned to produce the mass of Al determined in part (a)?
Figure 21-24
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





