By the method used to graph Figure 12-20, plot ln P versus 1/T for liquid white phosphorus,
Question:
By the method used to graph Figure 12-20, plot ln P versus 1/T for liquid white phosphorus, and estimate
(a) Its normal boiling point and
(b) Its enthalpy of vaporization, ΔvapH, in kJ mol-1. Vapor pressure data: 76.6 °C, 1 mmHg; 128.0 °C, 10 mmHg; 166.7 °C, 40 mmHg; 197.3 °C, 100 mmHg; 251.0 °C, 400 mmHg.
Figure 12-20
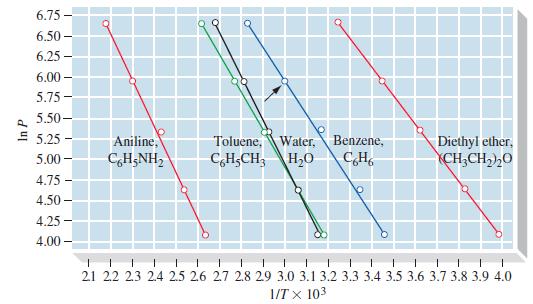
Transcribed Image Text:
6.75- 6.50- 6.25 6.00- 5.75- 5.50- E 5.25- 5.00- 4.75 4.50- 4.25- 4.00- - Q Aniline, CHẠNH, 09 Toluene, Water, C6H-CH3 H₂O Q Benzene, CH Diethyl ether, CH3CH₂)20 1 1 1 1 1 1 I 1 1 1 1 1 T 1 21 22 23 2.4 2.5 2.6 2.7 2.8 2.9 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 4.0 1/TX 103
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To plot ln P versus 1T for liquid white phosphorus we first ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Liquid nitrogen is stored in 0.5-m3 metal tanks that are thoroughly insulated. Consider the process of filling an evacuated tank, initially at 295 K. It is attached to a line containing liquid...
-
You are going to prepare a silicone polymer, and one of the starting materials is dichlorodimethylsilane, SiCl 2 (CH 3 ) 2 . You need its normal boiling point and to measure equilibrium vapor...
-
A horizontal spring is attached to the wall. The spring has a force constant k =1980 N/m and is compressed 0.08 m from its normal length. A block of mass 0.2kg is placed a rest against the spring....
-
The fieldwork for the 30 June 20X0 audit of Tracy Brewing Company Ltd was finished on 19 August 20X0 and the completed financial statements, accompanied by the signed audit reports, were mailed on 6...
-
Holly Hill Acres, Ltd. executed and delivered a promissory note and a purchase money mortgage to Rogers and Blythe. The note provided that it was secured by a mortgage on certain real estate and that...
-
Kiernan Construction Co. entered into a contract with Jackson for Jackson to build a house for her. Century Surety Co. executed a bond to protect Jackson from loss if Kiernan failed to construct the...
-
Assume that Hector Corporations comparative balance sheet reported these amounts: Requirement 1. Assume that on January 2, 2010, Hector sold 1/2 of its plant and equipment for $237,000 in cash....
-
Refer to the information in Exercise 10-4. Prepare a table showing depreciation and book value for each of the four years assuming double-declining-balance depreciation.
-
A production supervisor for Linkoln Company is comparing his budget to actuals for inventory components in May. He has asked you to prepare two documents which will be sent to the division finance...
-
. By completing this assignment, students will be demonstrating their knowledge and skills learnt from session 1-5 that include: o IP address planning o Local Area Network technologies o Implement...
-
One handbook lists the sublimation pressure of solid benzene as a function of Kelvin temperature, T, as log P (mmHg) = 9.846 - 2309/T. Another handbook lists the vapor pressure of liquid benzene as a...
-
Because solid p-dichlorobenzene, C 6 H 4 Cl 2 sublimes rather easily, it has been used as a moth repellent. From the data given, estimate the sublimation pressure of C 6 H 4 Cl 2 (s) at 25 C For C 6...
-
Justify each answer. In each part, A represents an n x n matrix. True or False. If U is m x n with orthogonal columns, then UU T x is the orthogonal projection of x onto Col U.
-
What career are you interested in pursuing? Hopefully, this activity will assist you. You will learn about various occupations that may interest you. This activity will guide you through the Bureau...
-
A company declared cash dividends of $0.20 per share. If there are 500,000 shares of common stock authorized, 100,000 shares issued, and 80,000 shares outstanding at the date of declaration, what is...
-
Let T: R2 R2 be given with respect to the standard basis by the matrix Find ker(T). ( }).
-
You have a diffraction grating apparatus with a variety of line densities and a 4 2 5 nm purple laser. You would like to produce a bright fringe at an angle of 3 3 from the right bisector. What are...
-
Cost of materials used $50,000 Direct labor costs 56,000 Factory overhead 28,000 Work in process inventory, beginning 45,000 Work in process inventory, ending 32,000 Determine the cost of goods...
-
One common approach that companies use to protect human rights is a supplier code of conduct. How can you increase the effectiveness of a code of conduct?
-
a. What is meant by the term tax haven? b. What are the desired characteristics for a country if it expects to be used as a tax haven? c. What are the advantages leading an MNE to use a tax haven...
-
Call Provisions TMCC has the right to buy back the securities on the anniversary date at a price established when the securities were issued (this feature is a term of this particular deal). What...
-
Time Value of Money would you be willing to pay $1.163 today in exchange for $10,000 in 30 years? What would be the key considerations in answering yes to no? Would your answer depend on who is...
-
Investment Comparison suppose that when TMCC offered the security for $1,163 the U.S. Treasury had offered an essentially identical security. Do you think it would have had a higher or lower price?...
-
The committee of a new golf club is setting the annual membership fee. The number of members depends on the membership fee charged and economic conditions. The forecast annual cash inflows from...
-
Pawan, who is self-employed, filed his tax return on November 1st and paid his $2500 balance owing. If the prescribed interest Pawan, who is self-employed, filed his tax return on November 1st and...
-
The responsibilities of Operations Management include obtaining all necessary inputs and drawing up a production plan that effectively uses the materials, capacity and knowledge available at the...

Study smarter with the SolutionInn App


