Calculate the aqueous solubility, in moles per liter, of each of the following. (a) BaCrO4, Ksp =
Question:
Calculate the aqueous solubility, in moles per liter, of each of the following.
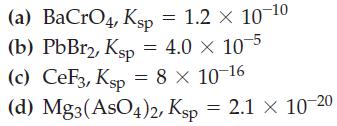
Transcribed Image Text:
(a) BaCrO4, Ksp = 1.2 x 10-10 (b) PbBr2, Ksp = 4.0 × 10-5 (c) CeF3, Ksp = 8 × 10-16 (d) Mg3(AsO4)2, Ksp = 2.1 × 10-20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The solubility product constant Ksp describes the equilibrium between the dissolved ions and the solid compound at saturation It can be used to calcul...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Calculate the solubility of each of the following compounds in moles per liter and grams per liter. (Ignore any acidbase properties.) a. Ag3PO4, Ksp = 1.8 10-18 b. CaCO3, Ksp = 8.7 10-9
-
What is the solubility in moles per liter of Ca(OH)2? Use data from Table 13.2.
-
What is the solubility in moles per liter of AgCl? Use data from Table 13.2.
-
Sandys Socks makes the worlds best socks. Information for the last eight months follows: Prepare a scatter graph by plotting Sandys data on a graph. Then draw a line that you believe best fits the...
-
Below are balance sheet and income statement data for Blue Panel Corporation. Additional information for Blue Panel Corporation is as follows: (a) Property, plant, and equipment with an original...
-
The New Branch School District operates a fleet of 40 buses that serve approximately 1,000 students in grades K12. The bus operation involves 30 regular routes, plus special routes for activities,...
-
William Stem filed an action against Gary Braden, seeking to rescind a contract for the sale of an automobile and to obtain the return of the purchase price plus interest. The trial court granted...
-
Marston Marble Corporation is considering a merger with the Conroy Concrete Company. Conroy is a publicly traded company, and its beta is 1.30. Conroy has been barely profitable, so it has paid an...
-
5. Let v = [1,0], w = [1,1]. Find the projector P that projects R onto (v) along (u). What are range(P) and null(P)?
-
Arrange the following solutes in order of increasing molar solubility in water: AgCN, AgIO 3 , AgI, AgNO 2 , Ag 2 SO 4 . Explain your reasoning.
-
The following K sp values are found in a handbook. Write the solubility product expression to which each one applies. For example, K sp( AgCl) = [Ag + ][Cl] = 1.8 x 10 -10 . (a) Kep(CrF3) = 6.6 10-1...
-
In Problems 2138, find the equation of the parabola described. Find the two points that define the latus rectum, and graph the equation. Vertex at (3, 0); focus at (3, -2)
-
Write a method activityCalories that takes a string indicating an activity (sit, walk, jog, bike, swim) and duration in minutes (integer), and returns the estimated calories expended (double)....
-
A stack emitting 65 g/s of SO2 has an effective stack height of 150 m. The windspeed is 4 m/s at 10 m above ground and 6 m/s at the effective smokestack height. It is a cloudy summer day. Estimate...
-
a FIRM WILL SPEND 100000 IMMEDIATELY TO IMPLEMENT A 3- YEAR CAPITAL PROYECT. tHE PROYECT IS EXPECTED TO PROVIDE OPERATING CASHFLOWS OF 100000 AT THE END OF A YEAR 1.225000 AT THE END OF YEAR 2 AND...
-
How to code a website with Eclipse using the code below Scanner scanner = new Scanner(System.in); while (true) { System.out.println("Exit? (y exits)"); String input = scanner.nextLine(); if...
-
how costs will be reported throughout the project, how the reports will be sent, what the reports will cover, and what the reporting frequency will be.
-
Smith-Kline Company maintains inventory records at selling prices as well as at cost. For 2011, the records indicate the following data: Required: Assuming the price level increased from 1.00 at...
-
The cost curve for the city water supply is C(Q) = 16 + 1/4 Q2, where Q is the amount of water supplied and C(Q) is the cost of providing Q acre-feet of water. (An acre-foot is the amount of water...
-
(a) What is the major rationale for the use of variable costing? (b) Discuss why variable costing may not be used for financial reporting purposes.
-
Monthly production costs in Pesavento Company for two levels of production are as follows. Indicate which costs are variable, fixed, and mixed, and give the reason for eachanswer. 3,000 units 6,000...
-
For Loder Company, the relevant range of production is 4080% of capacity. At 40% of capacity, a variable cost is $4,000 and a fixed cost is $6,000. Diagram the behavior of each cost within the...
-
Port 26. BOATING The light from a lighthouse can be seen from an 18-mile radius. A boat is anchored so that it can just see the light from the lighthouse. A second boat is located 25 miles from the...
-
For the beam illustrated in the figure, find the locations and magnitudes of the maximum tensile bending stress due to Mand the maximum shear stress due to V. Parameters are a = 310 mm, b = 160 mm, c...
-
(Related to Checkpoint 5.2) (Future value) Leslie Mosallam, who recently sold her Porsche, placed $10,400 in a savings account paying annual compound interest of 5 percent. a. Calculate the amount of...

Study smarter with the SolutionInn App


