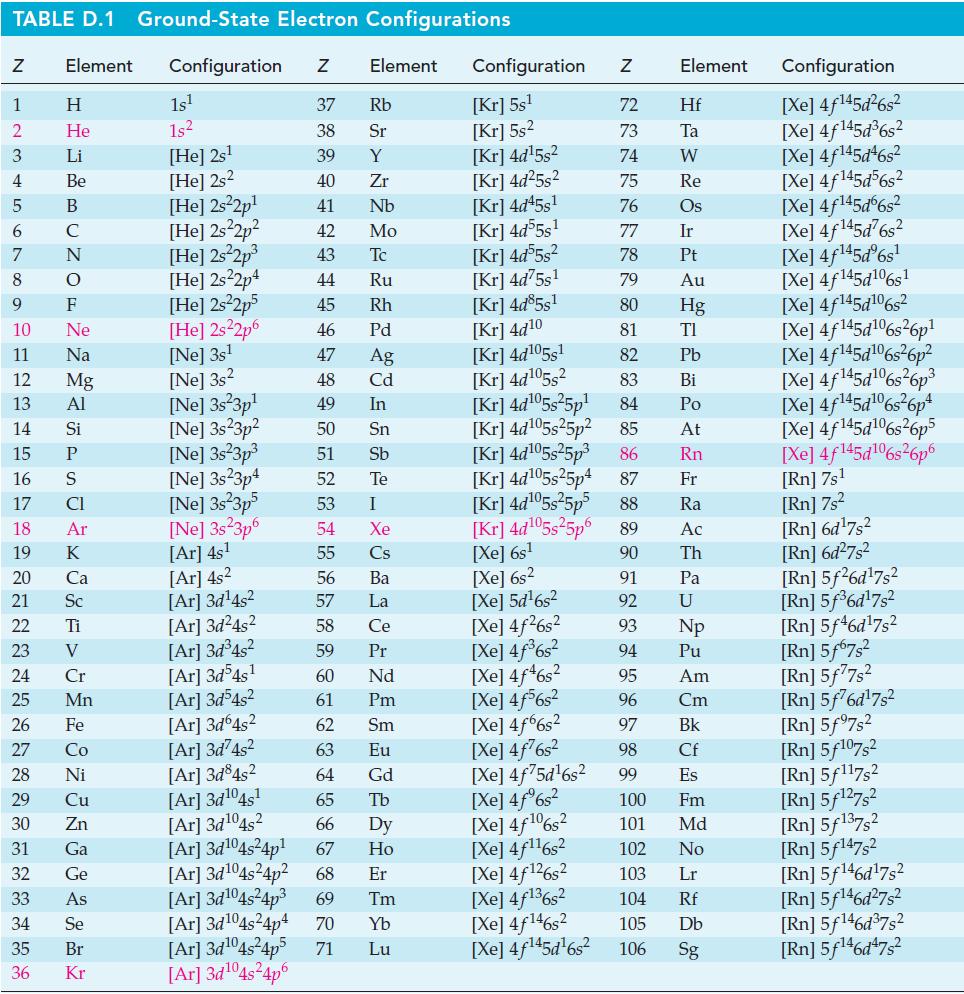
Calculate the entropy change, S, for the following processes. If necessary, look up required data in Appendix
Question:
Calculate the entropy change, ΔS, for the following processes. If necessary, look up required data in Appendix D.
(a) A mole of He(g) undergoes an expansion from V to 2V at 298 K.
(b) The temperature of one mole of CH4(g) is increased from 298 K to 325 K at a constant pressure of 1 bar.
Transcribed Image Text:
TABLE D.1 Ground-State Electron Configurations Element Configuration Z Z 1 2 3 4 5 6 7 8 9 10 11 12 13 14 16 17 18 19 24 25 HIGÅ LUZONS JY E> 0 ≤ 2 3 2 3 5 3 3 2 2 5 2 27 Η 15 P He 29 Li 30 Be 31 B 32 C 33 F Ne Na 20 Ca Mg 21 Sc Al 22 Ti Si 23 V CI Ar K 26 Fe 28 Ni Cr Mn Co Cu Zn Ga Ge As 34 Se 35 Br 36 Kr 1s¹ 1s² [He] 2s¹ [He] 2s2 [He] 2s²2p¹ [He] 2s²2p² [He] 2s²2p³ [He] 2s22p4 [He] 2s²2p5 [He] 2s²2p6 [Ne] 3s¹ [Ne] 3s2 [Ne] 3s 3p¹ [Ne] 3s23p² [Ne] 3s²3p³ [Ne] 3s23p4 [Ne] 3s²3p5 [Ne] 3s 3p6 [Ar] 4s¹ [Ar] 4s² [Ar] 3d¹4s² [Ar]3d²4s² [Ar] 3d³4s² [Ar] 3d54s¹ [Ar] 3d³4s² [Ar] 3d64s² Element 37 Rb 38 Sr 39 Y 40 Zr 41 Nb 42 Mo 43 Tc 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 Sn 51 Sb Te 52 53 54 55 56 57 58 59 60 61 62 63 64 I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd [Ar]3d²4s² [Ar] 3d845² [Ar] 3d¹04s¹ [Ar] 3d¹04s2 65 Tb 66 67 [Ar] 3d¹04s²4p¹ [Ar] 3d¹04s²4p² 68 Dy Ho Er [Ar] 3d¹04s²4p³ 69 Tm [Ar] 3d¹04s²4p4 70 Yb [Ar]3d¹04s²4p5 71 Lu [Ar]3d¹04s²4p6 Configuration Z [Kr] 5s¹ [Kr] 5s² [Kr] 4d¹5s² [Kr] 4d²5s² [Kr] 4d45s¹ [kr] 4d55s¹ [kr] 4d55s² [Kr] 4d75s¹ [Kr] 4d85s1 [Kr] 4d10 [Kr] 4d105s1 [kr] 4d¹05s² [kr] 4d¹05s²5p¹ [kr] 4d¹05s25p² [Kr] 4d¹05s²5p³ [kr] 4d¹05s25p4 [Xe] 6s² [Xe] 5d¹6s² [Xe] 4f²6s² [Xe] 4f³6s² [Xe] 4f46s2 [Xe] 4f6s2 [Xe] 4f6s2 [Xe] 4f²6s² [Xe] 4f75d¹6s² [Xe] 4f%s2 [Xe] 4f106s2 [Xe] 4f¹¹6s² NRNKERKR [Xe] 4f126s2 [Xe] 4f136s2 [Xe] 4f146s2 [Xe] 4f¹45d¹6s² 72 Hf 73 Ta W 74 75 Re 76 Os 77 Element 78 79 80 81 82 83 84 85 86 87 Fr [kr] 4d¹05s²5p5 88 Ra [Kr] 4d¹05s²5p6 89 Ac [Xe] 6s¹ 90 Th 91 92 93 94 95 96 97 98 99 Ir Pt Au Hg TI Pb Bi Po At Rn Pa U Np Pu Am Cm Bk Cf Es 100 Fm 101 Md 102 No 103 Lr 104 Rf 105 Db 106 Sg Configuration [Xe] 4f¹45d²6s² [Xe] 4f145d³6s² [Xe] 4f145d46s2 [Xe] 4f145d56s2 [Xe] 4f145d6s2 [Xe] 4f¹45d²6s² [Xe] 4f¹45dº6s¹ [Xe] 4f145d106s1 [Xe] 4f145d106s2 [Xe] 4f145d6s26p* [Xe] 4f145d106s36p? [Xe] 4f145d16s?6p3 [Xe] 4f145d6s®6p* [Xe] 4f145d16s26p5 [Xe] 4f145d106s26p6 [Rn] 7s¹ [Rn] 7s² [Rn] 6d¹7s² [Rn] 6d²7s² [Rn] 5f26d¹7s² [Rn] 5f³6d¹7s² [Rn] 5f46d¹7s2 [Rn] 5f67s² [Rn] 5f77s² [Rn] 5f76d¹7s² [Rn] 5f97s2 [Rn] 5f107,2 [Rn] 5f117s2 [Rn] 5f¹27s² [Rn] 5f137,2 [Rn] 5f147s2 Rn] 5f¹46d¹7s² [Rn] 5f¹46d²7s² [Rn] 5f¹46d³7s2 [Rn] 5f¹46d¹7s²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Entropy change for the expansion of a mole of Heg from V to 2V at 298 K Since helium is an ideal g...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Calculate the entropy change, S, for the following processes. If necessary, look up required data in Appendix D. (a) The pressure of one mole of O 2 (g) is increased from P to 2P at 298 K. (b) The...
-
3. Assume that you hold a well-diversified portfolio that has an expected return of 10.0% and a beta of 1.20. You are in the process of buying 1,000 shares of Bridge Corp at $10 a share and adding it...
-
Calculate the entropy change for a process in which 3.00 moles of liquid water at 0oC is mixed with 1.00 mole of water at 100.oC in a perfectly insulated container. (Assume that the molar heat...
-
Find the maximum of f(x,y) = x + y - x - y - xy
-
School costs money. Is this an expenditure that you should have avoided? A year of tuition at a public four-year college costs about $8,655, and a year of tuition at a public two-year college costs...
-
The production manager of a large Cincinnati manufacturing firm once made the statement, "I would like to use LP, but it's a technique that operates under conditions of certainty. My plant doesn't...
-
At the end of 2010, Zeman, Corp., had total assets of $25 million and total liabilities of $13 million. Included in the assets were property, plant, and equipment with a cost of $9 million and...
-
The following data are the actual results for Marvelous Marshmallow Company for August. Actual output.............................................................13,500 cases Actual variable...
-
Choose a country/destination and discuss the following: (3 points) (100 words or 1-2 slides) 1. Seasonality of the destination 2. List what their natural resources are in terms of food (and drinks if...
-
Estimate the normal boiling point of bromine, Br 2 in the following way: Determine vap H for Br 2 from data in Appendix D. Assume that vap H remains constant and that Troutons rule is obeyed. TABLE...
-
In Example 13-3, we dealt with vap H and vap S for water at 100 C. (a) Use data from Appendix D to determine values for these two quantities at 25 C. (b) From your knowledge of the structure of...
-
Describe as many ways as you can in which functions in imperative programming languages differ from functions in mathematics.
-
The estimated taxable income for the year ended 31 December 2021 is R8 425 000. What is the 1st provisional payment amount?
-
Solve for y. 2-2+15=39
-
Can you elaborate on the concept of political agency within the context of power dynamics, exploring how individuals and collectives navigate institutional constraints to effect meaningful change?
-
You, CPA, work in the tax department of a small public accounting firm in Edmonton, Alberta. The busy tax season has just finished and on a brisk Monday morning in May the partner introduces you to...
-
Question: d. ?During December, Ingrid Legal Services provided legal services, and the client prepaid $7,000. ?Ingrid Legal Services recorded this amount as Unearned Revenue. The job will take several...
-
Fleishman Corporation issued 5,000 shares of its no-par common stock for $ 28 per share and 510 shares of its $ 50 par value preferred stock for $ 52.50 per share. Prepare the journal entries to...
-
What is the order p of a B + -tree? Describe the structure of both internal and leaf nodes of a B + -tree.
-
Sigma Corporation applies overhead cost to jobs on the basis of direct labor cost. Job V, which was started and completed during the current period, shows charges of $5,000 for direct materials,...
-
Estimated cost and operating data for three companies for the upcoming year follow: Predetermined overhead rates are computed using the following allocation bases in the three companies: Required:...
-
The following information is taken from the accounts of Latta Company. The entries in the T-accounts are summaries of the transactions that affected those accounts during the year. The overhead that...
-
The owner of Kamisoto Company presented the following statement of profits for the year December 31, 2020. Kamisoto Statement of Profits Revenue from sales P80,500 Operating expenses: Salaries paid...
-
Wal-Mart's frustrations in India Seven years ago Wal-Mart set out to be India's top retailer by 2015. The business plan was called Project Jai Ho, a Hindi phrase meaning "let there be victory". But...
-
a) identify potential mitigating strategies the organization could employ to reduce the impact of their highest ranked social risks. (2 marks) Risk The effect on the community and environment due to...

Study smarter with the SolutionInn App


