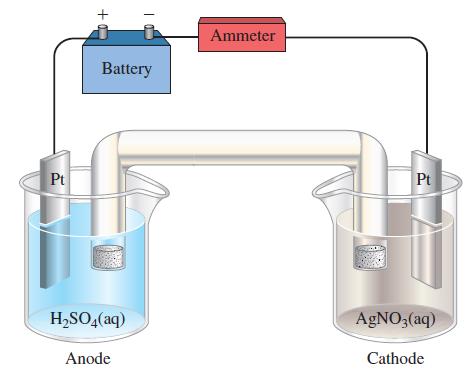
Electrolysis is carried out for 2.00 h in the following cell. The platinum cathode, which has a
Question:
Electrolysis is carried out for 2.00 h in the following cell. The platinum cathode, which has a mass of 25.0782 g, weighs 25.8639 g after the electrolysis. The platinum anode weighs the same before and after the electrolysis.
(a) Write plausible equations for the half-cell reactions that occur at the two electrodes.
(b) What must have been the magnitude of the current used in the electrolysis (assuming a constant current throughout)?
(c) A gas is collected at the anode. What is this gas, and what volume should it occupy if (when dry) it is measured at 23 °C and 755 mmHg pressure?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





