Explain why equation (17.10) fails when applied to dilute solutionsfor example, when you calculate the pH of
Question:
Explain why equation (17.10) fails when applied to dilute solutions—for example, when you calculate the pH of 0.010 M NaH2PO4.
Eq. 17.10
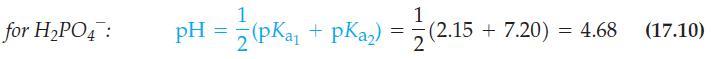
Transcribed Image Text:
for H₂PO4: 1 pH = (pKa₁ + pka₂) = 1 2 (2.15 (2.15 +7.20) = 4.68 (17.10)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Equation 1710 fails when applied to dilute solutions because it does not take into account the activ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Explain why Newtons method fails when applied to the equation 3x = 0 with any initial approximation x 0. Illustrate your explanation with a sketch.
-
Calculate the pH of each of the following solutions (Ka and Kb values are given in Appendix D): (a) 0.095 M propionic acid (C2H5COOH), (b) 0.100 M hydrogen chromate ion (HCrO4-, (c) 0.120 M pyridine...
-
Calculate the pH of a 1 L solution containing (a) 10 mL of 5 M NaOH, (b) 10 mL of 100 mM glycine and 20 mL of 5 M HCl, and (c) 10 mL of 2 M acetic acid and 5 g of sodium acetate (formula weight 82 g ...
-
Revenue for the new startup company "BCB Excavating" for the years 2017 through 2021 have been $543,000, $603,400, $789,000, $845,000, and $889,000 respectively. Year 2017 2018 2019 2020 2021 2022...
-
Mark each of the accounts listed in the following table as follows: a. In column (1), indicate in which statementincome statement (IS) or balance sheet (BS)the account belongs. b. In column (2),...
-
Fuqua Companys sales budget projects unit sales of part 198Z of 10,000 units in January, 12,000 units in February, and 13,000 units in March. Each unit of part 198Z requires 4 pounds of materials,...
-
Copy your worksheet from Question 6 into another worksheet. Change the increase from 10% to 18%. Protect the worksheet, so that changes cannot be made. Question 6 Open a new spreadsheet. Type...
-
Magic Enterprises borrowed $18,000 from the local bank on July 1, 2010, when the company was started. The note had an 8 percent annual interest rate and a one-year term to maturity. Magic Enterprises...
-
(a) Let3+ and 2 = a + bi be complex numbers. Suppose that 7 Argument = 12' find Argument(22). 7-2 (b) Let the map f: CC be defined by f(z) = Find f() if=1+2i. 1 (c) Solve the equation -12 i(9-2),...
-
A series of titrations of lactic acid, CH 3 CH(OH)COOH (pK a = 3.86) is planned. About 1.00 mmol of the acid will be titrated with NaOH(aq) to a final volume of about 100 mL at the equivalence point....
-
Complete the derivation of equation (17.10) outlined in Are You Wondering 17-1. Then derive equation (17.11). Eq. 17.10 Eq. 17.11 for HPO4: 1 pH = (pKa + pka) = 1 2 (2.15 (2.15 +7.20) = 4.68 (17.10)
-
Meo Consulting, Inc. completed the following transactions during February 2013, its first month of operations: Feb 2 Sold 6,500 shares for $65,000 to DaPing Meo to start the consulting practice. 3...
-
In June 2022, Gray Company recorded $400,000 in sales of washing machines consisting of with $320,000 of customers as accounts receivable and the remainder in cash. Rousseau Co. incurred and paid...
-
Marcel attends graduate school. For 2022, he received a Form 1098-T, Tuition Statement, showing tuition paid of $14,300. What is the maximum amount of the lifetime learning credit he can claim for...
-
To what account is the Manufacturing Summary account closed?
-
Discuss the auditor's responsibility for identifying and acting on events subsequent to the reporting date (you need to consider the three defined phases). You may support your answer accounting...
-
At Stonehenge the Heelstone marks where the Sun rises farthest north during the year. About what day would that be on a modern calendar?
-
What is securitization? What part did securitization play in the financial crisis of 2008? Provide some insights as to what caused the financial crisis of 2008. Discuss the role of securitization in...
-
List four items of financial information you consider to be important to a manager of a business that has been operating for a year.
-
Which one(s) of the following are assets traded in financial markets: (a) 6-month Libor (b) A 5-year Treasury bond (c) A FRA contract (d) A caplet (e) Returns on 30-year German Bonds (f) Volatility...
-
Suppose you are given the following information on the spot rate rt: The rt follows: dt =rt + rt dWr. The annual drift is = .01. 15This is the wise because the forward price, Ft], belt belong to the...
-
Suppose at time t = 0, you are given four default-free zero-coupon bond prices P(t, T) with maturities from 1 to 4 years: P(0, 1) = .94, P(0, 2) = .92, P(0, 3) = .87, P(0, 4) .80 (a) How can you fit...
-
Given X1 and X2 to be the roots of the quadratic equation 4x - 7x = 3 evaluate the following expression. 2 -+ 3 x1x2 7 + (x1+x2) Note: Input your answer as an integer or a fraction a/b in the lowest...
-
Let f(x)= x+2 and g(x)=x2-2. Determine the domain of the composite function. (Enter your answer using interval notation.) fog Find the composite function. (fog)(x)=
-
In 2022, vxtunarflagi hf. purchased shares worth 10,000 shares in Suurferir hf. for ISK 215,000 and classified the investment as a financial asset for sale. At year-end 2022, the fair value of the...

Study smarter with the SolutionInn App


