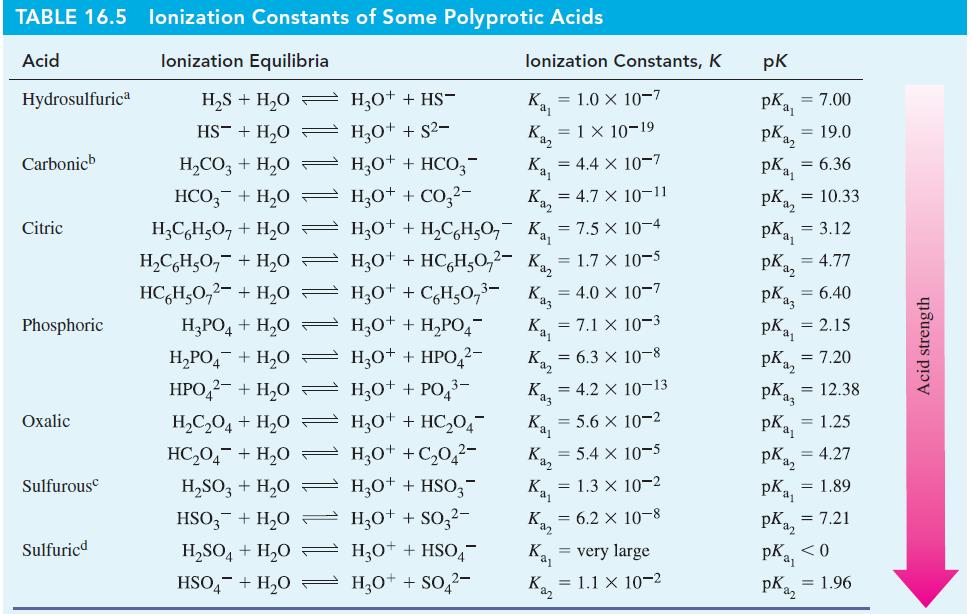
For 0.045 M H 2 CO 3 , a weak diprotic acid, calculate (a) [H 3 O
Question:
For 0.045 M H2CO3, a weak diprotic acid, calculate
(a) [H3O+],
(b) [HCO3-],
(c) [CO32-].
Use data from Table 16.5 as necessary.
Table 16.5
Transcribed Image Text:
TABLE 16.5 lonization Constants of Some Polyprotic Acids Acid lonization Equilibria H₂S + H₂O HS + H₂O Hydrosulfurica Carbonic Citric Phosphoric Oxalic Sulfurous Sulfuricd H₂CO3 + H₂O HCO3 + H₂O = H₂C6H₂O7 + H₂O = H₂C6H5O₂ + H₂0 HC H₂0₂²- + H₂0 H₂PO4+H₂O → H₂PO4+H₂0 H₂O+ + HS- H3O+ + S²- H3O+ + HCO3- Ka H₂O+ + CO3²- K₁ = 4.7 x 10-11 H₂O+ + H₂C6H5O₂K₁₁ = 7.5 x 10-4 H3O+ + HC6H₂O2- Ka = 1.7 x 10-5 H₂O+ + C₂H₂O₂³- H3O+ + H₂PO4 H30++ HPO4²- HPO4²- + H₂0 — H3O+ + PO4³- H₂C₂O4+H₂0 H3O+ + HC₂04 HC₂04 + H₂0 H3O+ +C₂04²- H₂SO3 + H₂O H3O+ + HSO3- HSO3 + H₂0 H3O+ + SO3²- lonization Constants, K Ka, = 1.0 x 10-7 Ka = 1 × 10-19 H₂SO4+H₂O H₂O+ + HSO4 HSO4+H₂O = H₂O+ + SO4²- Kaz K₂ = 4.4 x 10-7 K₁₁ = 7.1 x 10-3 K₁₂ = 6.3 × 10-8 = 4.0 x 10-7 Kaz = 4.2 X 10-13 K₁₁ = 5.6 x 10-2 Kaz Kaj = 5.4 x 10-5 = 1.3 x 10-2 = 6.2 × 10-8 Kaj Kaz = very large = 1.1 x 10-2 pk pka₁ pka₂ = 7.00 = 19.0 pk₁₁ pka2 = 10.33 pKa = 3.12 = 6.36 pK₂ = 4.77 = 2.15 pka3 = 6.40 pk ₁₁ pka2 = 7.20 = 12.38 pKaz pKay pKaz pka₁ pka₂ = 7.21 = 1.25 = 4.27 = 1.89 PK₁₁ <0 pKaz = 1.96 Acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Calculate H3O Step 1 Write the equilibrium constant equation for the first dissociation of H2CO3 H...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Carbonic acid is a weak diprotic acid (H 2 CO 3 ) with K a1 = 4.43 x 10 -7 and K a2 = 4.73 x 10 -11 . The equivalence points for the titration come at approximately pH 4 and 9. Suitable indicators...
-
Use data from Table to estimate ÎH for the combustion of methane (CH4), as shown below: Table s | 14 39 95 45 72 1 1419 6847064968 77386 42222 34 985 0302 121 Si H C O 437 490 9 31222241122...
-
For each of the following ions, write two equationsone showing its ionization as an acid and the other as a base: (a) HSO 3 - ; (b) HS - ; (c) HPO 4 - . Then use data from Table 16.5 to predict...
-
Explain why some conditions on the market are regarded as anomalies and explain how that affects factor investing.
-
During the past 2 years Meacham Industries issued three separate convertible bonds. For each of them, calculate the conversion price: a. A $1,000-par-value bond that is convertible into 10 shares of...
-
Why are data flow diagrams developed in a hierarchy? What are the names of some levels in the hierarchy?
-
Which of the following is an activity not usually associated with forensic accounting and fraud examination consulting and litigation support? 1. A. Assessing fraud risk associated with internal...
-
Len Kumar started his own consulting firm. Kumar Consulting on June 1, 2017 the trial balance at June 30 is as follows. In addition to those accounts listed on the trial balance, the chart of...
-
2. Home Depot (HD) recently paid a annual dividend of $1.50 per share. Using a one period dividend growth model, what is the price of one share of HD stock if the terminal dividend growth rate is 5%?...
-
Explain why [PO 4 3- ] in 1.00 M H 3 PO 4 is not simply 1 / 3 [H 3 O + ], but much, much less than 1 / 3 [H 3 O + ].
-
Continuing the dilutions described in Example 16-4, should we expect the percent ionization to be 13% in 0.0010 M CH 3 COOH and 42% in 0.00010 M CH 3 COOH? Explain. Example 16-4 What is the percent...
-
An Allowance for Doubtful Debts is created (A) When debtors become bankrupt (B) When debtors cease to be in business (C) To provide for possible bad debts (D) To write-off bad debts
-
Assume that you used the remainder method to convert a given decimal number D to hexadecimal. The following remainders were calculated and are listed in the order that they were calculated, i.e.,...
-
7. Last night Marion won $5000 in a lottery. She was given two options. She can take $5000 today or $X every 6 months (beginning 6 months from now) for 2 years. If the options are equivalent and the...
-
1. Consider the charge distribution shown, for which Q= 3.6C. Find the 1m +Q C a) force on the +2C in millinewtons 2m mN +2MC
-
Consider the following 4-table-join query: USE Northwind; SELECT S.ShipperID ,S.CompanyName ,O.OrderID ,O.ShippedDate ,EMP.EmployeeID ,EMP.LastName ,O.CustomerID ,C.CompanyName FROM Shippers AS S...
-
What is the range of the function f(x) = 3x + 2 over the interval -6 < x < 6?
-
J. Marcel Enterprises has gathered projected cash flows for two projects. At what interest rate would the company be indifferent between the twoprojects? Year Project I Project J -$250.000-$250.000...
-
Swifty company is a publicly held corporation whose $1 par value stock is actively traded at $30 per share. The company issued 3400 shares of stock to acquire land recently advertised at $93000. When...
-
The comparative accounts payable and long-term debt balances of a company are provided below. Based on this information, what is the amount and percentage of increase or decrease that would be shown...
-
The comparative temporary investments and inventory balances for a company are provided below. Based on this information, what is the amount and percentage of increase or decrease that would be shown...
-
Income statement information for Sheaf Corporation is provided below. Sales $500,000 Gross profit 140,000 Net income 40,000 Prepare a vertical analysis of the income statement for Sheaf Corporation.
-
(a) A velocity selector consists of electric and magnetic fields described by the expressions = E k and B = B , with B = 14.0 mT. Find the value of E (in kV/m) such that a 830 eV electron moving in...
-
Beginning Retained Earnings are $40,000 sales are $53,800 expenses are $44,000 and dividends paid are $2,900. How much is the net income or loss for the company?
-
A fixed charge Q= +2.13 C is held fixed at the origin of an xy plane. A second charge q= +2.982 C is released from rest at the xy coordinate of (+1.15 m, +0.670 m). (a) If the mass of q is 2.70 g,...

Study smarter with the SolutionInn App


