For the reaction below, r G = 27.07 kJ mol -1 at 298 K. Use this
Question:
For the reaction below, ΔrG° = 27.07 kJ mol-1 at 298 K.
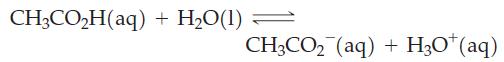 Use this thermodynamic quantity to decide in which direction the reaction is spontaneous when the concentrations of CH3CO2H(aq), CH3CO2-(aq), and H3O+(aq) are 0.10 M, 1.0 x 10-3 M, and 1.0 x 10-3 M, respectively.
Use this thermodynamic quantity to decide in which direction the reaction is spontaneous when the concentrations of CH3CO2H(aq), CH3CO2-(aq), and H3O+(aq) are 0.10 M, 1.0 x 10-3 M, and 1.0 x 10-3 M, respectively.
Transcribed Image Text:
CH3CO₂H(aq) + H₂O(1) CH3CO₂ (aq) + H₂O+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The standard Gibbs free energy change rG for the reaction CH3COHaq H2O1 CH3CO2 aq H3O aq is 2707 kJ ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
For the reaction below, r G = 29.05 kJ mol -1 at 298 K. Use this thermodynamic quantity to decide in which direction the reaction is spontaneous when the concentrations of NH 3 (aq), NH 4 + (aq),...
-
Use thermodynamic data at 298 K to decide in which direction the reaction is spontaneous when the partial pressures of SO 2 , O 2 , and SO 3 are 1.0 x 10 -4 , 0.20, and 0.10 bar, respectively. 2...
-
Use thermodynamic data at 298 K to decide in which direction the reaction is spontaneous when the partial pressures of H 2 , Cl 2 , and HCl are all 0.5 bar. Cl(g) 2 HCI(g) H(g) + Cl(g)
-
A company had average total assets of $500,000, gross sales of $575,000, and net sales of $550,000. The companys total asset turnover is a. 1.15. b. 1.10. c. 0.91. d. 0.87. e. 1.05.
-
Gundy Corporation produces area rugs. The following per unit cost information is available: direct materials $18, direct labor $9, variable manufacturing overhead $5, fixed manufacturing overhead $6,...
-
An investor has two bonds in her portfolio, Bond C and Bond Z. Each bond matures in 4 years, has a face value of $1,000, and has a yield to maturity of 9.6%. Bond C pays a 10% annual coupon, while...
-
As a gambling facility, MGM Desert Inn, Inc., regularly holds and executes negotiable instruments. During a period of two months, patron William E. Shack Jr. entered MGM and delivered eight checks to...
-
Ready-Set-Go Co. distributes suitcases to retail stores and extends credit terms of 1/10, n/30 to all of its customers. At the end of June, Ready-Set-Go's inventory consisted of suitcases costing...
-
Criticism of the World Bank is generally on a diverse range of issues but they generally centre around concern about the approaches adopted by the World Bank in formulating their policies, and the...
-
For the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g) all but one of the following equations is correct. Which is incorrect, and why? (a) K = K p ; (b) r S = ( r H - r G)/T; (c) K = e - r G/RT ; (d) r G...
-
In the synthesis of gaseous methanol from carbon monoxide gas and hydrogen gas, the following equilibrium concentrations were determined at 483 K: [CO(g)] = 0.0911 M, [H 2 (g)] = 0.0822 M, and 3[CH 3...
-
A 20 ft ladder leans against a building. Let x represent the distance between the building and the foot of the ladder, and let y represent the height the ladder reaches on the building. a. Write an...
-
The use of bribery in the business setting is an important ethical dilemma many companies face both domestically and abroad. The Bribe Payers Index is a study published every three years to assess...
-
Founded in 1966 by Pierre Bellon in France, Sodexo (sodexo.com) is the worldwide leader in providing a range of quality of life services, including workplace design, onsite food provision, facilities...
-
Can a company be good at corporate social responsibility but not be sustainability oriented? Is it possible to focus on sustainability but not corporate social responsibility? Based on reading the...
-
Neil Frasier, a friend of your family, is a very proficient accountant. Hes 61 and has spent his entire career working at the Sears Holdings Corp. headquarters in Chicago. He just got a call from a...
-
Do you think facilitating payments (speed payments) should be ethical? Does it matter in which country, or part of the world, such payments are made?
-
Suppose someone said to you, The only real measure of a salesperson is the amount of sales produced. How might you respond?
-
If the jobs displayed in Table 18.24 are processed using the earliestdue-date rule, what would be the lateness of job C? TABLE 18.24 Processing Times and Due Dates for Five Jobs Job C D E...
-
Multinational transfer pricing, global tax minimization. Industrial Diamonds, Inc., based in Los Angeles, has two divisions: South African Mining Division, which mines a rich diamond vein in South...
-
International transfer pricing, taxes, goal congruence. Argone Division of Gemini Corporation is located in the United States. Its effective income tax rate is 20%. Another division of Gemini,...
-
Transfer pricing, goal congruence. The Orsilo Corporation makes end sells 10,000 multisystem music players each year Its Assembly Division purchases components from other divisions of Orsilo or from...
-
Explain why felons should vote First, felons should be allowed to vote because it is their right as American citizens. It is their responsibility as citizens who have paid their debts to society and...
-
Case Study 1 Sears, Roebuck used to be the largest retailer in the United States, with sales representing 1 to 2 percent of the U.S. gross national product for almost 40 years after World War II....
-
1. If quadrilateral ABCD is a trapezoid then the segments of the diagonal are proportional. Prove, using the definition of a trapezoid, that the shaded pair of triangles is similar and the segments...

Study smarter with the SolutionInn App


