For the reaction if the H 2 O were obtained as a gas rather than a liquid,
Question:
For the reaction
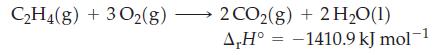
if the H2O were obtained as a gas rather than a liquid,
(a) Would the heat of reaction be greater (more negative) or smaller (less negative) than that indicated in the equation?
(b) Explain your answer.
(c) Calculate the value of ΔrH° in this case.
Transcribed Image Text:
C₂H4(g) + 3O2(g) 2 CO2(g) + 2 H₂O (1) A,H° -1410.9 kJ mol-1 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a More negative b The enthalpy of reaction is the difference in enth...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Suppose that inventory at the beginning of the year consists of 5 units of WIDGET01, costing $33. 00 each. During the year, you received three different shipments for WIDGET01 as follows: on January...
-
In a job order cost accounting system, what records serve as a subsidiary ledger for Goods in Process Inventory? For Finished Goods Inventory?
-
= 78, = 14, n = 49 Determine x and x from the given parameters of the population and the sample size.
-
What are the different types of schemes associated with complex frauds?
-
The CVP income statements shown below are available for Armstrong Company and Contador Company. Instructions (a) Compute the degree of operating leverage for each company and interpret your results....
-
explain diagram of ER model and relational model and attach their diagrams too Each cinema is identified by its name and has its residency at an address which consists of a street and city only....
-
The state of Kentucky filed a tax lien against Panbowl Energy, claiming unpaid taxes. Six months later, Panbowl bought a powerful drill from Whayne Supply, making a down payment of $11,500 and...
-
A calorimeter that measures an exothermic heat of reaction by the quantity of ice that can be melted is called an ice calorimeter. Now consider that 0.100 L of methane gas, CH 4 (g), at 25.0 C and...
-
Which of the following gases has the greater fuel value on a per liter (STP) basis? That is, which has the greater heat of combustion? (a) Coal gas: 49.7% H 2 , 29.9% CH 4 , 8.2% N 2 , 6.9% CO, 3.1%...
-
The speed of sound in an ideal gas _______. Select all that are correct: a. Depends on T where T is absolute temperature b. Depends on T where T is temperature in C c. Depends on k, where k = c p /c...
-
When investors and analysts were trying to value Twitter's stock for its November 7, 2013 IPO, they used a shorthand multiple in which each user of a social network was worth around $100. This...
-
How must the organisational policies and standards be modified to adjust for the new strategy?
-
Include the title of your Excel worksheet here and attach it to your Project Portfolio. Your Excel worksheet should include all the data and analysis.
-
Prepare the journal entry for the following exchange with NO commercial substance. You MUST show your computation of the gain recognized. Assume accumulated depreciation has been updated. Old...
-
What is the main difference between average physical and marginal physical product? Give an example of marginal physical property.
-
Match the items with the related statements that follow. a. Internal control b. A need of internal control c. Managements responsibility d. Independent accountants audit 1. Evaluates managements...
-
What steps must a business take to implement a program of social responsibility?
-
What interest rates should be used in determining the amount of interest to be capitalized? How should the amount of interest to be capitalized be determined?
-
How should the amount of interest capitalized be disclosed in the notes to the financial statements? How should interest revenue from temporarily invested excess funds borrowed to finance the...
-
Discuss the basic accounting problem that arises in handling each of the following situations. (a) Assets purchased by issuance of capital stock. (b) Acquisition of plant assets by gift or donation....
-
Find the per unit length resistance of a cylinder with a radius of a = 2 cm, made of copper with a conductivity of = 5.998 x 107 [S/m] (shown in Figure 1). Figure 1: 3-D view of a cylinder made of...
-
Does global criminal activity operates under the substantive criminal law and procedural criminal law concepts?
-
Consider a server used in information technologies at MIT. on the system Response times (milliseconds) were recorded for 20 consecutive transactions. a. Obtain the frequency distribution for these...

Study smarter with the SolutionInn App


