Here is a way to test the validity of the statement made in conjunction with the three
Question:
Here is a way to test the validity of the statement made in conjunction with the three key ideas governing the ionization of polyprotic acids. Determine the pH of 0.100 M succinic acid in two ways: first by assuming that H3O+ is produced only in the first ionization step, and then by allowing for the possibility that some H3O+ is also produced in the second ionization step. Compare the results, and discuss the significance of your finding.
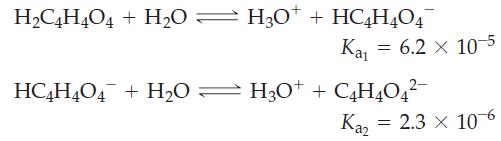
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





