How much heat, in kilojoules, is evolved in the complete combustion of (a) 1.325 g C 4
Question:
How much heat, in kilojoules, is evolved in the complete combustion of
(a) 1.325 g C4H10(g) at 25 °C and 1 atm;
(b) 28.4 L C4H10(g) at STP;
(c) 12.6 L C4H10(g) at 23.6 °C and 738 mmHg?
Assume that the enthalpy of reaction does not change significantly with temperature or pressure. The complete combustion of butane, C4H10(g), is represented by the equation
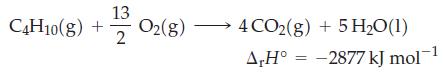
Transcribed Image Text:
C4H10(g) + O₂(g) 13 2 4 CO2(g) + 5H₂0 (1) A,H° -2877 kJ mol™
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To solve this problem we will use the following steps Calculate the moles of butane Mo...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A company is considering a 5-year project that opens a new product line and requires an initial outlay of $77,000. The assumed selling price is $98 per unit, and the variable cost is $60 per unit....
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Randy, Inc., can issue 3-month commercial paper with a face value of $1,500,000 for $1,450,000. Transaction costs will be $1,500. The effective annualized percentage cost of the financing, based on a...
-
Describe the relations among the income statement, the manufacturing statement, and a detailed listing of factory overhead costs.
-
A jet plane is speeding down the runway during takeoff. Air resistance is not negligible. Describe a situation. For each problem, draw a motion diagram, a force identification diagram, and a free...
-
Douglas Singletary purchased a mobile home from P&A Investments, Inc. d/b/a Andys Mobile Home and Land Sales. On November 17, 2007, Singletary entered in a sales agreement with Andys to purchase a...
-
PCB Corporation manufactures a single product. Monthly production costs incurred in the manufacturing process are shown below for the production of 3,000 units. The utilities and maintenance costs...
-
Map the EER model of the following databases, to a relational model. Show all integrity types(Clarify your answer by underline the Primary key, and dotted line the Foreign Key. ( Member SSN {PK}...
-
m 30C Haze n 4. Question 4 An online store wants to analyze various aspects of using coupons, including the distribution of discounts and savings. The company is developing a report that displays the...
-
The standard enthalpy of reaction for the combustion of octane is rH = -5.48 x 10 3 kJ/mol C 8 H 18 (l). How much heat, in kilojoules, is liberated per gallon of octane burned? (Density of octane =...
-
What is the final temperature (in C) of 1.24 g of water with an initial temperature of 20.0 C after 6.052 J of heat is added to it?
-
Nestlé's financial statements are presented in Appendix B. Financial statements of Petra Foods are presented in Appendix C. Instructions (a) Based on the information contained in these...
-
How does American culture conceptualize and delineate standards of beauty and handsomeness?
-
Compare the difference between regional versus global integration. b) Which one will be the most important in the future and explain why. Provide examples to support the arguments. References after...
-
How has the proliferation of technology, particularly communication technology, influenced the processes of cultural diffusion and cultural retention? Can you provide examples illustrating these...
-
Consider that 65% of a company's assets were financed with debt. If the cost of debt is 25% EA and the cost of equity is 29.5% EA; What is the weighted cost of the resources with which the company...
-
In a essay, explain why you want to be a teacher. Include past experiences, people, or intrinsic/extrinsic rewards that have led you to this decision.
-
Using the data that follows and assuming cost of goods sold is $273,700, prepare the cost of goods sold section of a merchandising income statement (periodic inventory system). Include the amount of...
-
Evaluate the integral, if it exists. Jo y(y + 1) dy
-
At a recent executive committee meeting, the controller for Ricardo Company remarked, With only a single key difference between U.S. GAAP and iGAAP for property, plant, and equipment, it should be...
-
What is a modified accelerated cost recovery system (MACRS)? Speculate as to why this system is now required for tax purposes.
-
Fernandez Corporation purchased a truck at the beginning of 2010 for $50,000. The truck is estimated to have a salvage value of $2,000 and a useful life of 160,000 miles. It was driven 23,000 miles...
-
Carl delivers papers for spending money. He gets $10 for each day that he works in addition to $0.50 for each paper that he delivers. If Carl works on Monday and gets paid $25, how many papers did he...
-
Stock A's stock has a beta of 1.30, and its required return is 16.00%. Stock B's beta is 0.80. If the risk-free rate is 4.75%, what is the required rate of return on B's stock? ( Hint: First find the...
-
A learning plan that meets individual and group training and development needs will be collaboratively developed, agreed to, and implemented. Draw on your own experience and that of others, plus...

Study smarter with the SolutionInn App


