In 2013, the IUPAC recommended that the atomic masses of 12 elements be expressed as an atomic
Question:
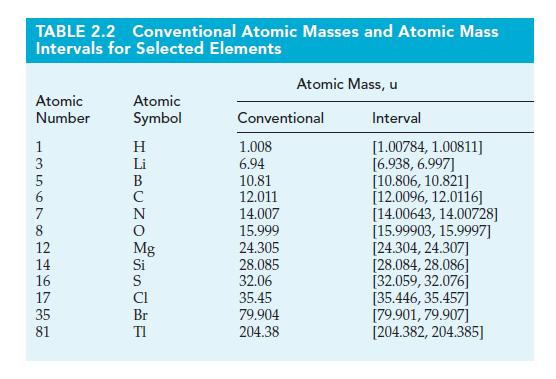
In 2013, the IUPAC recommended that the atomic masses of 12 elements be expressed as an atomic mass interval rather as a single invariant value. (See Section 2-5 and Table 2.2.) For example, the IUPAC recommends that the atomic mass of Cl be given as [35.446, 35.457]. Consequently, the results of calculations involving the atomic mass of chlorine should, in principle, be reported as a range of values. Demonstrate this approach by calculating the range of values possible for the mass percent of silver in an impure sample if all the silver in a 26.39 g sample is converted to 31.56 g of silver chloride.
Table 2.2
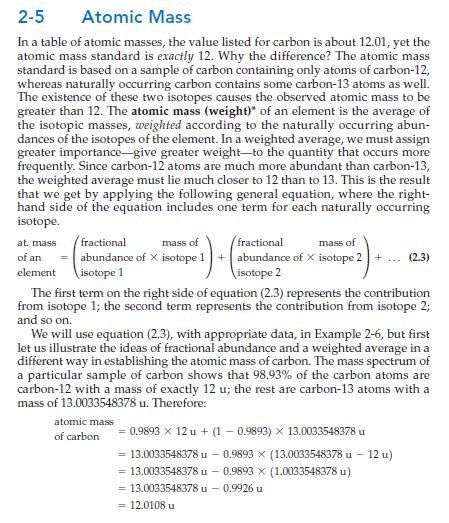
Section 2-5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





