In biochemical reactions* the phosphorylation of amino acids is an important step. Consider the following two reactions
Question:
In biochemical reactions* the phosphorylation of amino acids is an important step. Consider the following two reactions and determine whether the phosphorylation of arginine with ATP is spontaneous.
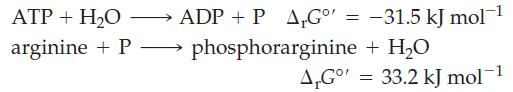
Transcribed Image Text:
ADP + P A₁Gº' = -31.5 kJ mol-¹ phosphorarginine + H₂O ATP + H₂O arginine + P→→→→→→ A,Go' = 33.2 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine whether the phosphorylation of arginine with ATP is spontaneous we need to compare the ...View the full answer

Answered By

Shaira grace
I have experience of more than ten years in handing academic tasks and assisting students to handle academic challenges. My level of education and expertise allows me communicate eloquently with clients and therefore understanding their nature and solving it successfully.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The racemization of amino acids is an important reaction in a number of bacteria. This is a pyridoxal-phosphate catalyzed reaction. Outline a curved-arrow mechanism for this reaction showing clearly...
-
Behind terrorism is a complex endeavor, shaped by various factors and objectives unique to each terrorist group. In Max Abrahms article, "What Terrorists Really Want: Terrorist Motives and...
-
Proteins are made up of individual molecular units of unique structure known as amino acids. The order or sequence of amino acids is an important factor in determining protein structure and function....
-
For each of the following tests, identify two different samples of people who would have the expertise to serve as subject matter experts (SMEs) for providing judgments regarding the content validity...
-
Urbina Inc. is preparing its annual budgets for the year ending December 31, 2014. Accounting assistants furnish the following data. An accounting assistant has prepared the detailed manufacturing...
-
An astronaut wishes to visit the Andromeda galaxy, making a one-way trip that will take 30.0 yr in the spacecrafts frame of reference. Assume that the galaxy is 2.00 x 106 ly away and that the...
-
Although the attributes sampling plans introduced in this chapter are accurate portrayals of plans applied in practice, they are by necessity generic. Over the years, professional journals such as...
-
Wright Company employs a computer-based data processing system for maintaining all company records. The current system was developed in stages over the past five years and has been fully operational...
-
If WHO, the World Health Organization,defines health as a state of completephysical, mental and social well-being and not merely the absenceof disease and infirmity (WHO, 2011)and wellness is...
-
Following are some standard Gibbs energies of formation, f G, at 1000 K: NiO(s), -115 kJ mol -1 ; MnO(s), -280 kJ mol -1 ; TiO 2 (s), -630 kJ mol -1 . The standard Gibbs energy of formation of CO(g)...
-
For the reaction N 2 O 4 (g) 2 NO 2 (g), r H = +57.2 kJ mol -1 and K = 0.113 at 298 K. (a) What is K at 0 C? (b) At what temperature will K = 1.00?
-
Related party transactions are an every-day occurrence. What is so special about them that standard setters formulate rules for the disclosure?
-
Quality control involves testing units and determining if they are within the specifications for the final product. The purpose of the testing is to determine any needs for corrective actions in the...
-
Consider a portfolio consisting of the three risky stocks. You decide to invest 25 percent in Apple, 35 percent in HP and 40 percent in Spree. These stocks show the volatility at the level of 11.15...
-
Draw out the mechanism for the reaction involving butan-2-one with one of the two reagents shown below: primary amine or alcohol. You only need to show one mechanism. To assist you, there are two...
-
The operations vice president of Security Home Bank is investigating the efficiency of the bank's operations. She is concerned about the costs of handling routine transactions at the bank and would...
-
The management team of Blue Industries was evaluating its performance for the first half of the year. Production and sales of its fans were on budget at 3,000 units to date, with the following income...
-
Michael is a new employee in the financial reporting department of Goldberg Corporation, a mid-size publicly held corporation with annual revenues of $75 million. As Goldberg Corporation prepared for...
-
An 8.0 kg crate is pulled 5.0 m up a 30 incline by a rope angled 18 above the incline. The tension in the rope is 120 N, and the crates coefficient of kinetic friction on the incline is 0.25. a. How...
-
Production-Volume Variance Analysis and Sales Volume Variance. Dawn Floral Creations, Inc., makes jewelry in the shape off flowers. Each piece is hand-made and takes an average of 1.5 hours to...
-
Comprehensive review of Chapters working backward from given variances. The Mancusco Company uses a flexible budget and standard costs to aid planning and control of its machining manufacturing...
-
The Beal Manufacturing Companys costing system has two direct-cost categories: direct materials and direct manufacturing labor. Manufacturing overhead (both variable and fixed) is allocated to...
-
How do transposable elements impact genome stability and evolution, and what mechanisms do cells employ to regulate their activity and prevent genomic instability ?
-
What role does RNA editing play in expanding the functional diversity of the transcriptome, and how might dysregulation of RNA editing contribute to disease pathogenesis ?
-
On January 1, 2023, Bertrand, Incorporated, paid $86,900 for a 40 percent interest in Chestnut Corporation's common stock. This investee had assets with a book value of $229,500 and liabilities of...

Study smarter with the SolutionInn App


