In some cases, the titration curve for a mixture of two acids has the same appearance as
Question:
In some cases, the titration curve for a mixture of two acids has the same appearance as that for a single acid; in other cases it does not.
(a) Sketch the titration curve (pH versus volume of titrant) for the titration with 0.200 M NaOH of 25.00 mL of a solution that is 0.100 M in HCl and 0.100 M in HNO3. Does this curve differ in any way from what would be obtained in the titration of 25.00 mL of 0.200 M HCl with 0.200 M NaOH? Explain.
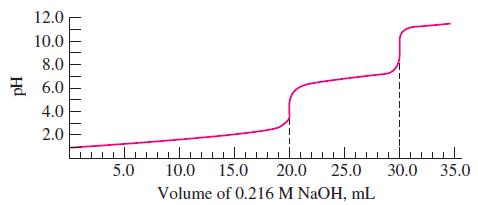
(b) The titration curve shown was obtained when 10.00 mL of a solution containing both HCl and H3PO4 was titrated with 0.216 M NaOH. From this curve, determine the stoichiometric molarities of both the HCl and the H3PO4.
(c) A 10.00 mL solution that is 0.0400 M H3PO4 and 0.0150 M NaH2PO4 is titrated with 0.0200 M NaOH. Sketch the titration curve.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




