In the equilibrium described in Example 15-12, the percent dissociation of N 2 O 4 can be
Question:
In the equilibrium described in Example 15-12, the percent dissociation of N2O4 can be expressed as
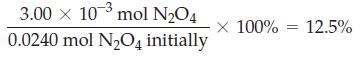
What must be the total pressure of the gaseous mixture if N2O4(g) is to be 10.0% dissociated at 298 K?
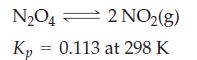
(for pressures in atmospheres).
Example 15-12
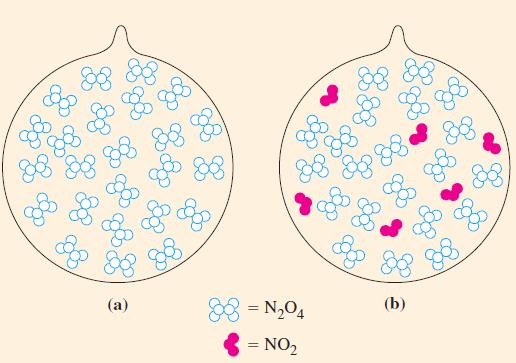
A 0.0240 mol sample of N2O4(g) is allowed to come to equilibrium with NO2(g) in a 0.372 L flask at 25 °C. Calculate the amount of N2O4 present at equilibrium (Fig. 15-9).
![]()
Figure 15-9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





