In the gas phase, iodine reacts with cyclopentene (C 5 H 8 ) by a free radical
Question:
In the gas phase, iodine reacts with cyclopentene (C5H8) by a free radical mechanism to produce cyclopentadiene (C5H6) and hydrogen iodide. Explain how each of the following affects the amount of HI(g) present in the equilibrium mixture in the reaction
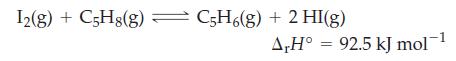
(a) Raising the temperature of the mixture;
(b) Introducing more C5H6(g) at constant volume;
(c) Doubling the volume of the container holding the mixture;
(d) Adding an appropriate catalyst;
(e) Adding an inert gas such as He to a constant-volume reaction mixture.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





